Abstract
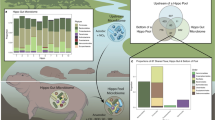
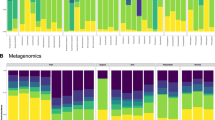
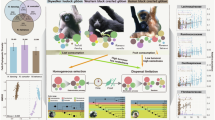
The term core microbiome describes microbes that are consistently present in a particular habitat. If the conditions in that habitat are highly variable, core microbes may also be considered to be ecological generalists. However, little is known about whether metabolic competition and microbial interactions influence the ability of some microbes to persist in the core microbiome while others cannot. We investigated microbial communities at three sites in the guts of European seabass under four dietary conditions. We identified generalist core microbial populations in each gut site that are shared across fish, present under multiple diets and persistent over time. We found that core microbes tend to show synergistic growth in co-culture, and low levels of predicted and validated metabolic competition. Within core microbial species, we found high levels of intraspecific variability and strain-specific habitat specialization. Thus, both intraspecific variability and interspecific facilitation may contribute to the ecological stability of the animal core microbiome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shabat, S. K. B. et al. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 10, 2958–2972 (2016).
Friedman, N. et al. Diet‐induced changes of redox potential underlie compositional shifts in the rumen archaeal community. Environ. Microbiol. 19, 174–184 (2017).
Zeevi, D. et al. Structural variation in the gut microbiome associates with host health. Nature 568, 43–48 (2019).
Kayser, J., Schreck, C. F., Yu, Q., Gralka, M. & Hallatschek, O. Emergence of evolutionary driving forces in pattern-forming microbial populations. Proc. R. Soc. B 373, 20170106 (2018).
Donaldson, G. P., Lee, S. M. & Mazmanian, S. K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32 (2016).
Jeraldo, P. et al. Quantification of the relative roles of niche and neutral processes in structuring gastrointestinal microbiomes. Proc. Natl Acad. Sci. USA 109, 9692–9698 (2012).
Shaani, Y., Zehavi, T., Eyal, S., Miron, J. & Mizrahi, I. Microbiome niche modification drives diurnal rumen community assembly, overpowering individual variability and diet effects. ISME J. 12, 2446–2457 (2018).
Ghoul, M. & Mitri, S. The ecology and evolution of microbial competition. Trends Microbiol. 24, 833–845 (2016).
Pacheco, A. R., Moel, M. & Segrè, D. Costless metabolic secretions as drivers of interspecies interactions in microbial ecosystems. Nat. Commun. 10, 103 (2019).
Travisano, M. & Rainey, P. B. Studies of adaptive radiation using model microbial systems. Am. Nat. 156, S35–S44 (2000).
Bolnick, D. I., Svanbäck, R., Araújo, M. S. & Persson, L. Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc. Natl Acad. Sci. USA 104, 10075–10079 (2007).
Shade, A. & Handelsman, J. Beyond the Venn diagram: the hunt for a core microbiome. Environ. Microbiol. 14, 4–12 (2011).
Thomas, T. et al. Diversity, structure and convergent evolution of the global sponge microbiome. Nat. Commun. 7, 11870 (2016).
Astudillo-García, C. et al. Evaluating the core microbiota in complex communities: a systematic investigation. Environ. Microbiol. 19, 1450–1462 (2017).
Coyte, K. Z., Schluter, J. & Foster, K. R. The ecology of the microbiome: networks, competition, and stability. Science 350, 663–666 (2015).
Bastolla, U. et al. The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 458, 1018–1020 (2009).
Thébault, E. & Fontaine, C. Stability of ecological communities and the architecture of mutualistic and trophic networks. Science 329, 853–856 (2010).
Lurgi, M., Montoya, D. & Montoya, J. M. The effects of space and diversity of interaction types on the stability of complex ecological networks. Theor. Ecol. 9, 3–13 (2016).
Bolnick, D. I. & Doebeli, M. Sexual dimorphism and adaptive speciation: two sides of the same ecological coin. Evolution 57, 2433–2449 (2003).
Van Valen, L. Morphological variation and width of ecological niche. Am. Nat. 99, 377–390 (1965).
Hacquard, S. et al. Microbiota and host nutrition across plant and animal kingdoms. Cell Host Microbe 17, 603–616 (2015).
Wilson, J. & Castro, L. Morphological diversity of the gastrointestinal tract in fishes. Fish. Physiol. 30, 1–55 (2010).
Lanan, M. C., Rodrigues, P. A. P., Agellon, A., Jansma, P. & Wheeler, D. E. A bacterial filter protects and structures the gut microbiome of an insect. ISME J. 10, 1866–1876 (2016).
Godoy-Vitorino, F. et al. Comparative analyses of foregut and hindgut bacterial communities in hoatzins and cows. ISME J. 6, 531–541 (2012).
Zhang, Z. et al. Spatial heterogeneity and co-occurrence patterns of human mucosal-associated intestinal microbiota. ISME J. 8, 881–893 (2014).
Looft, T. et al. Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations. ISME J. 8, 1566–1576 (2014).
Sriswasdi, S., Yang, C.-c & Iwasaki, W. Generalist species drive microbial dispersion and evolution. Nat. Commun. 8, 1162 (2017).
Levins, R. Evolution in Changing Environments: Some Theoretical Explorations (Princeton Univ. Press, 1968).
Shade, A. et al. Conditionally rare taxa disproportionately contribute to temporal changes in microbial diversity. mBio 5, e01371-14 (2014).
Kreimer, A., Doron-Faigenboim, A., Borenstein, E. & Freilich, S. NetCmpt: a network-based tool for calculating the metabolic competition between bacterial species. Bioinformatics 28, 2195–2197 (2012).
Cordero, O. X. et al. Ecological populations of bacteria act as socially cohesive units of antibiotic production and resistance. Science 337, 1228–1231 (2012).
Bolnick, D. I. Intraspecific competition favours niche width expansion in Drosophila melanogaster. Nature 410, 463–466 (2001).
Costa, G. C., Mesquita, D. O., Colli, G. R. & Vitt, L. J. Niche expansion and the niche variation hypothesis: does the degree of individual variation increase in depauperate assemblages? Am. Nat. 172, 868–877 (2008).
Muller, E. E. L. Determining microbial niche breadth in the environment for better ecosystem fate predictions. mSystems 4, e00080-19 (2019).
Soule, M. & Stewart, B. R. The “niche-variation” hypothesis: a test and alternatives. Am. Nat. 104, 85–97 (1970).
Dennison, M. D. & Baker, A. J. Morphometric variability in continental and Atlantic island populations of chaffinches (Fringilla coelebs). Evolution 45, 29–39 (1991).
Feinsinger, P. & Swarm, L. A. “Ecological release,” seasonal variation in food supply, and the hummingbird Amazilia tobaci on Trinidad and Tobago. Ecology 63, 1574–1587 (1982).
Meiri, S., Dayan, T. & Simberloff, D. Variability and sexual size dimorphism in carnivores: testing the niche variation hypothesis. Ecology 86, 1432–1440 (2005).
Lister, B. C. & McMurtrie, R. E. On size variation in anoline lizards. Am. Nat. 110, 311–314 (1976).
Konstantinidis, K. T. & Tiedje, J. M. Genomic insights that advance the species definition for prokaryotes. Proc. Natl Acad. Sci. USA 102, 2567–2572 (2005).
Zaneveld, J. R., Lozupone, C., Gordon, J. I. & Knight, R. Ribosomal RNA diversity predicts genome diversity in gut bacteria and their relatives. Nucleic Acids Res. 38, 3869–3879 (2010).
Chaffron, S., Rehrauer, H., Pernthaler, J. & von Mering, C. A global network of coexisting microbes from environmental and whole-genome sequence data. Genome Res. 20, 947–959 (2010).
Roeselers, G. et al. Evidence for a core gut microbiota in the zebrafish. ISME J. 5, 1595–1608 (2011).
Sun, H., Jami, E., Harpaz, S. & Mizrahi, I. Involvement of dietary salt in shaping bacterial communities in European sea bass (Dicentrarchus labrax). Sci. Rep. 3, 1558 (2013).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581 (2016).
Wang, Q., Garrity, G. M., Tiedje, J. M. & Cole, J. R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267 (2007).
McDonald, D. et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6, 610–618 (2012).
Jing, X. et al. The bacterial communities in plant phloem-sap-feeding insects. Mol. Ecol. 23, 1433–1444 (2014).
Lozupone, C. & Knight, R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71, 8228–8235 (2005).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 (2010).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
Colwell, R. K. & Futuyma, D. J. On the measurement of niche breadth and overlap. Ecology 52, 567–576 (1971).
Roughgarden, J. Theory of Population Genetics and Evolutionary Ecology: An Introduction (1979).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Aziz, R. K. et al. The RAST Server: rapid annotations using subsystems technology. BMC Genom. 9, 75 (2008).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000).
Gryta, A., Frąc, M. & Oszust, K. The application of the Biolog EcoPlate approach in ecotoxicological evaluation of dairy sewage sludge. Appl. Biochem. Biotechnol. 174, 1434–1443 (2014).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7, 335–336 (2010).
Ward, D. M. et al. Genomics, environmental genomics and the issue of microbial species. Heredity 100, 207–219 (2008).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 26, 1641–1650 (2009).
Faust, K. & Raes, J. CoNet app: inference of biological association networks using Cytoscape. F1000res 5, 1519 (2016).
Acknowledgements
The research described here was supported by grants from the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant no. 640384), the Israel Science Foundation (grant no. 1947/19) and the Chief Scientist of the Ministry of Agriculture and Rural Development (grant no. 356-0665-14).
Author information
Authors and Affiliations
Contributions
F.K. conducted the experiments, molecular work and sequencing, analysed and interpreted the sequencing and molecular data, and wrote the manuscript. G.S. analysed some of the sequencing data. J.F. analysed some of the sequencing data and provided advice on analysis. S.E. conducted some of the microbiology experiments and isolation. O.O. consulted on the interpretation of the data. S.H. and A.C. helped with animal experiments and with the supervision and financial support of the project. I.M. obtained the funding, supervised the study, analysed and interpreted the data, financially managed the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–19, Supplementary Figs. 1–14 and Supplementary References.
Supplementary Data for Supplementary Table 16
Median richness of strains per part is presented in the table along with the P values after comparison of the strain richness between the different gut parts (n = 36 fish per part). Empty cells indicate non-significant P values for the one-sided tests performed (as it is indicated below the Wilcoxon P values in the table). The average occurrence and median abundance is also calculated in the table for the different parts, showing no significant differences in the abundances of these OTUs. The right two columns show the average abundance of strains that show niche expansion patterns compared with the strains that do not show the patterns.
Supplementary Data for Supplementary Table 17
Clusters with 97% sequence similarity of microbes, showing major and minor sequences per OTU, as described in the ‘Quantification of sequencing noise’ section in the Methods.
Supplementary Data 1
Quantitative PCR from microbial interactions using specific primers. The growth results are expressed in copy number μl−1 (using specific primers for each microbe) and the experiment was performed in triplicate.
Rights and permissions
About this article
Cite this article
Kokou, F., Sasson, G., Friedman, J. et al. Core gut microbial communities are maintained by beneficial interactions and strain variability in fish. Nat Microbiol 4, 2456–2465 (2019). https://doi.org/10.1038/s41564-019-0560-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0560-0