Abstract
After severe brain injury, it can be difficult to determine the state of consciousness of a patient, to determine whether the patient is unresponsive or perhaps minimally conscious1, and to predict whether they will recover. These diagnoses and prognoses are crucial, as they determine therapeutic strategies such as pain management, and can underlie end-of-life decisions2,3. Nevertheless, there is an error rate of up to 40% in determining the state of consciousness in patients with brain injuries4,5. Olfaction relies on brain structures that are involved in the basic mechanisms of arousal6, and we therefore hypothesized that it may serve as a biomarker for consciousness7. Here we use a non-verbal non-task-dependent measure known as the sniff response8,9,10,11 to determine consciousness in patients with brain injuries. By measuring odorant-dependent sniffing, we gain a sensitive measure of olfactory function10,11,12,13,14,15. We measured the sniff response repeatedly over time in patients with severe brain injuries and found that sniff responses significantly discriminated between unresponsive and minimally conscious states at the group level. Notably, at the single-patient level, if an unresponsive patient had a sniff response, this assured future regaining of consciousness. In addition, olfactory sniff responses were associated with long-term survival rates. These results highlight the importance of olfaction in human brain function, and provide an accessible tool that signals consciousness and recovery in patients with brain injuries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Respiration data that support the findings of this study are available from GitLab (https://gitlab.com/liorg/OlfactorySniffingAnalysis/). Source data for Figs. 1–4 are provided with the paper.
Code availability
Custom code created and used in this study is available from GitLab (https://gitlab.com/liorg/OlfactorySniffingAnalysis/).
References
Giacino, J. T. et al. Comprehensive systematic review update summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 91, 461–470 (2018).
Giacino, J. T., Fins, J. J., Laureys, S. & Schiff, N. D. Disorders of consciousness after acquired brain injury: the state of the science. Nat. Rev. Neurol. 10, 99–114 (2014).
Thibaut, A., Schiff, N., Giacino, J., Laureys, S. & Gosseries, O. Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol. 18, 600–614 (2019).
Stender, J. et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet 384, 514–522 (2014).
Schnakers, C. et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 9, 35 (2009).
Price, J. L. in The Human Nervous System (ed. Paxinos, G) (Academic, 1990).
Merrick, C., Godwin, C. A., Geisler, M. W. & Morsella, E. The olfactory system as the gateway to the neural correlates of consciousness. Front. Psychol. 4, 1011 (2014).
Mainland, J. & Sobel, N. The sniff is part of the olfactory percept. Chem. Senses 31, 181–196 (2006).
Wachowiak, M. All in a sniff: olfaction as a model for active sensing. Neuron 71, 962–973 (2011).
Frank, R. A., Dulay, M. F. & Gesteland, R. C. Assessment of the sniff magnitude test as a clinical test of olfactory function. Physiol. Behav. 78, 195–204 (2003).
Johnson, B. N., Mainland, J. D. & Sobel, N. Rapid olfactory processing implicates subcortical control of an olfactomotor system. J. Neurophysiol. 90, 1084–1094 (2003).
Arzi, A. et al. Humans can learn new information during sleep. Nat. Neurosci. 15, 1460–1465 (2012).
Rozenkrantz, L. et al. A mechanistic link between olfaction and autism spectrum disorder. Curr. Biol. 25, 1904–1910 (2015).
Arzi, A., Rozenkrantz, L., Holtzman, Y., Secundo, L. & Sobel, N. Sniffing patterns uncover implicit memory for undetected odors. Curr. Biol. 24, R263–R264 (2014).
Reden, J., Draf, C., Frank, R. A. & Hummel, T. Comparison of clinical tests of olfactory function. Eur. Arch. Otorhinolaryngol. 273, 927–931 (2016).
Rojas-Líbano, D. & Kay, L. M. Interplay between sniffing and odorant sorptive properties in the rat. J. Neurosci. 32, 15577–15589 (2012).
Johnson, B. N., Russell, C., Khan, R. M. & Sobel, N. A comparison of methods for sniff measurement concurrent with olfactory tasks in humans. Chem. Senses 31, 795–806 (2006).
Arzi, A. et al. Olfactory aversive conditioning during sleep reduces cigarette-smoking behavior. J. Neurosci. 34, 15382–15393 (2014).
Giacino, J. T. & Kalmar, K. Coma Recovery Scale-Revised: Administration and Scoring Guidelines. https://www.tbims.org/combi/crs/CRS%20Syllabus.pdf (JFK Johnson Rehabilitation Institute and New Jersey Neuroscience Institute, JFK Medical Center, 2004).
Rappaport, M., Dougherty, A. M. & Kelting, D. L. Evaluation of coma and vegetative states. Arch. Phys. Med. Rehabil. 73, 628–634 (1992).
Bareham, C. A. et al. Longitudinal bedside assessments of brain networks in disorders of consciousness: case reports from the field. Front. Neurol. 9, 676 (2018).
Wannez, S., Heine, L., Thonnard, M., Gosseries, O. & Laureys, S. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann. Neurol. 81, 883–889 (2017).
Bareham, C. A. et al. Longitudinal assessments highlight long-term behavioural recovery in disorders of consciousness. Brain Commun. 1, fcz017 (2019).
Linacre, J. M., Heinemann, A. W., Wright, B. D., Granger, C. V. & Hamilton, B. B. The structure and stability of the functional independence measure. Arch. Phys. Med. Rehabil. 75, 127–132 (1994).
Rowe, T. B., Macrini, T. E. & Luo, Z.-X. Fossil evidence on origin of the mammalian brain. Science 332, 955–957 (2011).
Sela, L. & Sobel, N. Human olfaction: a constant state of change-blindness. Exp. Brain Res. 205, 13–29 (2010).
Keller, A. Attention and olfactory consciousness. Front. Psychol. 2, 380 (2011).
Stevenson, R. J. Phenomenal and access consciousness in olfaction. Conscious. Cogn. 18, 1004–1017 (2009).
Nigri, A. et al. Central olfactory processing in patients with disorders of consciousness. Eur. J. Neurol. 23, 605–612 (2016).
Arzi, A. et al. The influence of odorants on respiratory patterns in sleep. Chem. Senses 35, 31–40 (2010).
Forgacs, P. B. et al. Preservation of electroencephalographic organization in patients with impaired consciousness and imaging-based evidence of command-following. Ann. Neurol. 76, 869–879 (2014).
Fernández-Espejo, D. et al. A role for the default mode network in the bases of disorders of consciousness. Ann. Neurol. 72, 335–343 (2012).
Noirhomme, Q., Brecheisen, R., Lesenfants, D., Antonopoulos, G. & Laureys, S. “Look at my classifier’s result”: disentangling unresponsive from (minimally) conscious patients. Neuroimage 145, 288–303 (2017).
Majdan, M. et al. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health 1, e76–e83 (2016).
Aidinoff, E. et al. Vegetative state outcomes improved over the last two decades. Brain Inj. 32, 297–302 (2018).
Monti, M. M. et al. Willful modulation of brain activity in disorders of consciousness. N. Engl. J. Med. 362, 579–589 (2010).
Coleman, M. R. et al. Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness. Brain 132, 2541–2552 (2009).
Napolitani, M. et al. Transcranial magnetic stimulation combined with high-density EEG in altered states of consciousness. Brain Inj. 28, 1180–1189 (2014).
Engemann, D. A. et al. Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain 141, 3179–3192 (2018).
Bekinschtein, T. A. et al. Classical conditioning in the vegetative and minimally conscious state. Nat. Neurosci. 12, 1343–1349 (2009).
Green, P., Rohling, M. L., Iverson, G. L. & Gervais, R. O. Relationships between olfactory discrimination and head injury severity. Brain Inj. 17, 479–496 (2003).
Sobel, N. et al. An impairment in sniffing contributes to the olfactory impairment in Parkinson’s disease. Proc. Natl Acad. Sci. USA 98, 4154–4159 (2001).
Plotkin, A. et al. Sniffing enables communication and environmental control for the severely disabled. Proc. Natl Acad. Sci. USA 107, 14413–14418 (2010).
Haviv, L. et al. Using a sniff controller to self-trigger abdominal functional electrical stimulation for assisted coughing following cervical spinal cord lesions. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 1461–1471 (2017).
Charland-Verville, V. et al. Detection of response to command using voluntary control of breathing in disorders of consciousness. Front. Hum. Neurosci. 8, 1020 (2014).
Owen, A. Into the Gray Zone: A Neuroscientist Explores the Border Between Life and Death (Simon and Schuster, 2017).
Kondziella, D., Friberg, C. K., Frokjaer, V. G., Fabricius, M. & Møller, K. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 87, 485–492 (2016).
Borer-Alafi, N., Gil, M. Sazbon, L. & Korn, C. Loewenstein communication scale for the minimally responsive patient. Brain Inj. 16, 593–609 (2002).
Seel, R. T. et al. Assessment scales for disorders of consciousness: evidence-based recommendations for clinical practice and research. Arch. Phys. Med. Rehabil. 91, 1795–1813 (2010).
Rosenthal, R. in The Handbook of Research Synthesis (eds Cooper, H. & Hedges, L. V.) 231–244 (Russell Sage Foundation, 1994).
Cliff, N. Dominance statistics: ordinal analyses to answer ordinal questions. Psychol. Bull. 114, 494–509 (1993).
Kim, H.-Y. Statistical notes for clinical researchers: chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 42, 152–155 (2017).
Acknowledgements
We thank the patients, their families and their caregivers for their cooperation; and O. Perl, L. Rösler and A. Elite for discussions. Work in the Sobel laboratory is supported by the Rob and Cheryl McEwen Fund for Brain Research. A.A. is supported by the Blavatnik Family Foundation, a Royal Society – Kohn International fellowship (NF150851) and an European Molecular Biology Organization (EMBO) fellowship (ALTF 33-2016).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.A. and N.S.; data collection: A.A., L.R. and L.G.; analysis: A.A., L.R., L.G., A.R. and N.S.; funding acquisition: A.A., N.S. and Y.S.; investigation: A.A., L.R., D.R. and Y.H.; methodology: A.A., L.R., L.G., A.R. and N.S.; project administration: A.A., L.R., D.R., Y.H., G.C., T.G., B.-Z.K., A.O., E.A., Y.S. and N.S.; resources: A.A., L.R., L.G., D.R., Y.H., G.C., T.G., B.-Z.K., A.O., E.A., Y.S. and N.S.; software: A.A., L.R. and L.G.; supervision: A.A., T.A.B., T.G., B.-Z.K., A.O., E.A., Y.S. and N.S.; validation: A.A., L.R. and L.G.; visualization: A.A. and N.S.; writing of the original draft: A.A. and N.S.; writing, review and editing: A.A., L.R., L.G., D.R., Y.H., T.A.B., G.C., T.G., B.-Z.K., A.O., E.A., Y.S. and N.S.
Corresponding authors
Ethics declarations
Competing interests
The findings of this manuscript are being used by the Weizmann Institute Office of Technology Licensing (Yeda) for the submission of a patent for a method of the detection of consciousness.
Additional information
Peer review information Nature thanks Hakwan Lau, Johan Lundström, Nicholas Schiff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
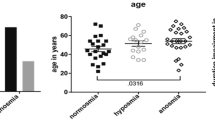
Extended Data Fig. 1 The sniff response reflects the current level of consciousness in patients with DOC.
Data are displayed by odorant and sniff. a–i, Normalized sniff volume after pleasant odorants (a, d, g), unpleasant odorants (b, e, h) and blank (c, f, i) during UWS (U) sessions (outline; n = 73) and MCS (M) sessions (filled; n = 73) for the first (a–c), second (d–f) and third (g–i) sniff after stimulus delivery. Left, each dot represents a session; flat violin plots show the distribution; the red lines denote the median; and the dashed horizontal lines denote the baseline value at 1 NFU. Right, data are the mean ± s.e.m. for each distribution. The P values beneath the distribution denotes its difference from baseline inhalation. The P values above the distributions denote the difference in sniff response across groups. P values were calculated using two-tailed Wilcoxon signed-rank tests for within-group comparisons and Wilcoxon rank-sum tests for between-group comparisons corrected for multiple comparisons. Corrected P values are indicated by an asterisk (*) and uncorrected P values are indicated by a hash (#) symbol (see Methods). *P < 0.05; #P < 0.05. Further analyses are provided in the Supplementary Information.
Extended Data Fig. 2 The sniff response to pure olfactory odorants in patients with DOC.
To estimate whether the effects that we observed were dependent on the contribution of the trigeminal nerve, we exposed a subset of patients to pure olfactory odorants and observed the replication of the effects. The normalized sniff volume after exposure to the pure olfactants of the pleasant odorant (phenylethyl alcohol; a, c, e) and unpleasant odorant (decanoic acid; b, d, f) during UWS sessions (outline; pleasant, n = 56; unpleasant, n = 57) and MCS sessions (filled; pleasant, n = 56; unpleasant n = 56) for the first (a, b), second (c, d) and third (e, f) sniff after odorant delivery. Left, each dot represents a session; flat violin plots show the distribution; the red lines denote the median; and the dashed horizontal lines denote the baseline value at 1 NFU. Right, data are the mean ± s.e.m. for each distribution. The P value beneath the distribution denotes its difference from baseline inhalation—that is, the existence of a sniff response. *P < 0.05; two-tailed Wilcoxon test. Further analyses are provided in the Supplementary Information.
Extended Data Fig. 3 The sniff response is similar with and without tracheostomy.
Normalized sniff volume after pleasant (a, c, e) or unpleasant (b, d, f) odorants during MCS sessions with (W; n = 44) and without (O; n = 29) tracheostomy for the first (a, b), second (c, d) and third (e, f) sniff after odorant delivery. Left, each dot represents a session; flat violin plots show the distribution; the red lines denote the median; and the dashed horizontal lines denote the baseline value at 1 NFU. Right, data are the mean ± s.e.m. for each distribution. The P value beneath the distribution denotes its difference from baseline inhalation—that is, the existence of a sniff response. *P < 0.05; two-tailed Wilcoxon test. About 60% of MCS sessions and 80% of UWS sessions were conducted in patients with a tracheostomy. Although tracheostomy significantly reduces nasal airflow, a measurable portion of nasal airflow remains. For example, we note that the raw data in Fig. 1c, d were obtained with a tracheostomy. Further analyses are provided in the Supplementary Information.
Extended Data Fig. 4 The sniff response during MCS sessions.
The red lines denote the sniff-response threshold (more than 15% change in magnitude and/or 0.35 s.d.): dots within the lines (white background) reflect sessions without a sniff response; dots beyond the lines (shaded background) reflect sessions with a sniff response. a–c, Each dot is a MCS session. a, Pleasant odorant. b, Unpleasant odorant. c, Blank. d, Percentage of patients in a MCS (not sessions) with sniff responses (white, 64.5%) and without sniff responses (red, 35.5%) across all three conditions.
Extended Data Fig. 5 The sniff response is prognostic for the recovery of consciousness and long-term survival in patients with DOC.
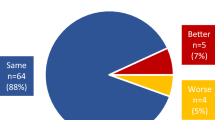
Data are shown by patient rather than by session. The red lines denote the threshold of a sniff response (more than 15% change in magnitude and/or 0.35 s.d.). a–c, Each dot is a session with the strongest sniff response of a patient in a MCS (n = 19). a, Pleasant odorant. b, Unpleasant odorant. c, Blank. d, Percentage of patients in a MCS with sniff responses (white, 64.5%) and without sniff responses (red, 35.5%) across all three conditions. e–g, Each dot is a session with the strongest sniff response of a patient with UWS; empty dots represent patients who later recovered (transitioned to MCS; n = 16) and filled dots represent patients who did not recover and remain unconscious (n = 8). e, Pleasant odorant. f, Unpleasant odorant. g, Blank. h, Percentage of patients with UWS who later transitioned to MCS (left, recovered) and who remain unconscious (right, unrecovered) with sniff responses (white; recovered, 62.5%; unrecovered, 0%) and without sniff responses (red; recovered, 37.5%; unrecovered, 100%) across all three conditions. i–k, Each dot is a patient with DOC (MCS and UWS; n = 43); filled black dots represent patients who died during the study and coloured dots represent patients who survived during the study (mean ± s.d., 37.3 ± 14.1 months after brain injury). i, Pleasant odorant. j, Unpleasant odorant. k, Blank. l, Percentage of patients with DOC with sniff responses (left) who survived (white, 91.7%) and who are deceased (D) (red, 8.3%) and of patients with DOC without sniff responses who survived (white, 36.8%) and are deceased (red, 63.2%). m–o, Relation between the functional independence measure and normalized sniff volume. Each dot is a patient with UWS who survived during the study (n = 12). m, Pleasant odorant. n, Unpleasant odorant. o, Blank. r represents Spearman correlation.
Extended Data Fig. 6 Dependence of the predictive value on sniff-response thresholds in patients with UWS.
a, The receiver-operating characteristic (ROC) for a range of sniff-response volume thresholds for a sniff-response volume variability threshold of 0.35. b, The ROC for a range of sniff-response volume variability thresholds for a sniff-response volume threshold of 0.85 (15% reduction). c, Distance from a randomized predictor for a range of sniff-response volume thresholds for a sniff-response volume variability threshold of 0.35. d, Distance from randomized predictor for a range of sniff-response volume variability thresholds for a sniff-response volume threshold of 0.85. e, Area under the curve (AuC) for different sniff-response volume variability thresholds. f, Area under the curve for different sniff-response volume thresholds. n = 24. Further analyses are provided in the Supplementary Information.
Extended Data Fig. 7 Dependence of the sensitivity and specificity on sniff-response thresholds.
a, Sensitivity (true-positive rate (TPR)) for a range of sniff-response volume and sniff-response volume variability thresholds. b, Specificity (true-negative rate (TNR)) for a range of sniff-response volume and sniff-response volume variability thresholds. c, Distance from a randomized predictor for a range of sniff-response volume and sniff-response volume variability thresholds. Further analyses are provided in the Supplementary Information.
Extended Data Fig. 8 Dependence of the predictive value on the relation between conditions.
a, ROC for a range of differences in the sniff-response volume between the unpleasant odorant and the blank. b, The true-positive and true-negative rates for a range of differences in the sniff-response volume between the unpleasant odorant and the blank. c, ROC for a range of differences in the sniff-response volume between the pleasant odorant and the blank. d, The true-positive and true-negative rates for a range of differences in the sniff-response volume between the pleasant odorant and the blank. e, ROC for a range of differences in the sniff-response volume between the pleasant and unpleasant odorants. f, The true-positive and true-negative rates for a range of differences in the sniff-response volume between the pleasant and unpleasant odorants. n = 24. Further analyses are provided in the Supplementary Information.
Supplementary information
Supplementary Information
This file contains control analyses, one supplementary table, and associated text.
Rights and permissions
About this article
Cite this article
Arzi, A., Rozenkrantz, L., Gorodisky, L. et al. Olfactory sniffing signals consciousness in unresponsive patients with brain injuries. Nature 581, 428–433 (2020). https://doi.org/10.1038/s41586-020-2245-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2245-5