Abstract
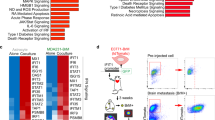
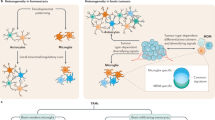
Brain metastasis still encompass very grim prognosis and therefore understanding the underlying mechanisms is an urgent need toward developing better therapeutic strategies. We uncover the intricate interactions between recruited innate immune cells and resident astrocytes in the brain metastatic niche that facilitate metastasis of melanoma and breast cancer. We show that granulocyte-derived lipocalin-2 (LCN2) induces inflammatory activation of astrocytes, leading to myeloid cell recruitment to the brain. LCN2 is central to inducing neuroinflammation as its genetic targeting or bone-marrow transplantation from LCN2−/− mice was sufficient to attenuate neuroinflammation and inhibit brain metastasis. Moreover, high LCN2 levels in patient blood and brain metastases in multiple cancer types were strongly associated with disease progression and poor survival. Our findings uncover a previously unknown mechanism, establishing a central role for the reciprocal interactions between granulocytes and astrocytes in promoting brain metastasis and implicate LCN2 as a prognostic marker and potential therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Source data for Figs. 1–7 and Extended Data Figs. 1–7 have been provided as Source Data files.
All other data supporting the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Eichler, A. F. et al. The biology of brain metastases-translation to new therapies. Nat. Rev. Clin. Oncol. 8, 344–356 (2011).
Cagney, D. N. et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 19, 1511–1521 (2017).
Takei, H., Rouah, E. & Ishida, Y. Brain metastasis: clinical characteristics, pathological findings and molecular subtyping for therapeutic implications. Brain Tumor Pathol. 33, 1–12 (2016).
Doron, H., Pukrop, T. & Erez, N. A blazing landscape: neuroinflammation shapes brain metastasis. Cancer Res. 79, 423–436 (2019).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Wu, A. M. L. et al. Aging and CNS myeloid cell depletion attenuate breast cancer brain metastasis. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-21-1549 (2021).
Quail, D. F. & Joyce, J. A. The microenvironmental landscape of brain tumors. Cancer Cell 31, 326–341 (2017).
Schulz, M., Salamero-Boix, A., Niesel, K., Alekseeva, T. & Sevenich, L. Microenvironmental regulation of tumor progression and therapeutic response in brain metastasis. Front. Immunol. 10, 1713 (2019).
Klemm, F. et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 181, 1643–1660 (2020).
Valiente, M. et al. The evolving landscape of brain metastasis. Trends Cancer. 4, 176–196 (2018).
Chen, Q. et al. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498 (2016).
Doron, H. et al. Inflammatory activation of astrocytes facilitates melanoma brain tropism via the CXCL10-CXCR3 signaling axis. Cell Rep. 28, 1785–1798 (2019).
Priego, N. et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med. 24, 1024–1035 (2018).
Wasilewski, D., Priego, N., Fustero-Torre, C. & Valiente, M. Reactive astrocytes in brain metastasis. Front. Oncol. 7, 298 (2017).
Zou, Y. et al. Polyunsaturated fatty acids from astrocytes activate pparγ signaling in cancer cells to promote brain metastasis. Cancer Discov. 9, 1720–1735 (2019).
Hirata, E. et al. The brain microenvironment induces DNMT1 suppression and indolence of metastatic cancer cells. iScience. 23, 101480 (2020).
Zhang, L. et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527, 100–104 (2015).
Gril, B. et al. Reactive astrocytic S1P3 signaling modulates the blood–tumor barrier in brain metastases. Nat. Commun. 9, 2705 (2018).
Schwartz, H. et al. Incipient melanoma brain metastases instigate astrogliosis and neuroinflammation. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-16-0485 (2016).
Flo, T. H. et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921 (2004).
Rathore, K. I. et al. Lipocalin 2 plays an immunomodulatory role and has detrimental effects after spinal cord injury. J. Neurosci. 31, 13412–13419 (2011).
Jang, E. et al. Phenotypic polarization of activated astrocytes: the critical role of lipocalin-2 in the classical inflammatory activation of astrocytes. J Immunol. 191, 5204–5219 (2013).
Jang, E. et al. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J. 27, 1176–1190 (2013).
Suk, K. Lipocalin-2 as a therapeutic target for brain injury: an astrocentric perspective. Prog. Neurobiol. 144, 158–172 (2016).
Stelzer, G. et al. VarElect: the phenotype-based variation prioritizer of the GeneCards Suite. BMC Genomics 17(Suppl 2), 444 (2016).
Lee, S. et al. Lipocalin-2 Is a chemokine inducer in the central nervous system: role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J. Biol. Chem. 286, 43855–43870 (2011).
Yan, L., Borregaard, N., Kjeldsen, L. & Moses, M. A. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL): modulation of MMP-9 activity by NGAL*. J. Biol. Chem. 276, 37258–37265 (2001).
Fernández, C. A. et al. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin. Cancer Res. 11, 5390–5395 (2005).
Kubben, F. J. G. M. et al. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur. J. Cancer 43, 1869–1876 (2007).
Nuntagowat, C., Leelawat, K. & Tohtong, R. NGAL knockdown by siRNA in human cholangiocarcinoma cells suppressed invasion by reducing NGAL/MMP-9 complex formation. Clin. Exp. Metastasis 27, 295–305 (2010).
Devireddy, L. R., Gazin, C., Zhu, X. & Green, M. R. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 123, 1293–1305 (2005).
Cabedo Martinez, A. I. et al. Biochemical and structural characterization of the interaction between the siderocalin NGAL/LCN2 (neutrophil gelatinase-associated lipocalin/lipocalin 2) and the N-terminal domain of its endocytic receptor SLC22A17. J. Biol. Chem. 291, 2917–2930 (2016).
Liddelow, S. A. & Barres, B. A. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 46, 957–967 (2017).
Proescholdt, M. A. et al. The management of brain metastases-systematic review of neurosurgical aspects. Cancers https://doi.org/10.3390/cancers13071616 (2021).
Schag, C. C., Heinrich, R. L. & Ganz, P. A. Karnofsky performance status revisited: reliability, validity, and guidelines. J. Clin. Oncol. 2, 187–193 (1984).
Blank, A. et al. Microglia/macrophages express alternative proangiogenic factors depending on granulocyte content in human glioblastoma. J. Pathol. 253, 160–173 (2021).
Khan, S. et al. Role of neutrophils and myeloid-derived suppressor cells in glioma progression and treatment resistance. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21061954 (2020).
Santiago-Sanchez, G. S. et al. Biological functions and therapeutic potential of lipocalin 2 in cancer. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21124365 (2020).
Tong, Z. et al. Neutrophil gelatinase-associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 68, 6100–6108 (2008).
Yang, J. et al. Lipocalin 2 promotes breast cancer progression. Proc. Natl Acad. Sci. USA. 106, 3913–3918 (2009).
Bauvois, B. & Susin, S. A. Revisiting neutrophil gelatinase-associated lipocalin (NGAL) in cancer: saint or sinner? Cancers https://doi.org/10.3390/cancers10090336 (2018).
Yang, J., McNeish, B., Butterfield, C. & Moses, M. A. Lipocalin 2 is a novel regulator of angiogenesis in human breast cancer. FASEB J. 27, 45–50 (2013).
Tyagi, A. et al. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat. Commun. 12, 474 (2021).
Rodvold, J. J., Mahadevan, N. R. & Zanetti, M. Lipocalin 2 in cancer: when good immunity goes bad. Cancer Lett. 316, 132–138 (2012).
Olson, B. et al. Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat. Commun. 12, 2057 (2021).
Jha, M. K. et al. Diverse functional roles of lipocalin-2 in the central nervous system. Neurosci. Biobehav. Rev. 49, 135–156 (2015).
Llorens, F. et al. Cerebrospinal fluid lipocalin 2 as a novel biomarker for the differential diagnosis of vascular dementia. Nat. Commun. 11, 619 (2020).
Chi, Y. et al. Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science 369, 276–282 (2020).
Erez, N., Truitt, M., Olson, P., Arron, S. T. & Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 17, 135–147 (2010).
Shani, O. et al. Fibroblast-derived IL33 facilitates breast cancer metastasis by modifying the immune microenvironment and driving type 2 immunity. Cancer Res. 80, 5317–5329 (2020).
Birnboim-Perach, R., Grinberg, Y., Vaks, L., Nahary, L. & Benhar, I. Production of stabilized antibody fragments in the E. coli bacterial cytoplasm and in transiently transfected mammalian cells. Methods Mol. Biol. 1904, 455–480 (2019).
Acknowledgements
The authors thank Y. Zilberstein from the Faculty Cellular and Molecular Imaging Center for her help with intracardiac injections and mouse imaging. We thank the Rabin Medical Center Biobank and A. Levy-Barda for their support of this research. We also thank E. Vollmer, E. Ostermeier and K. Kronenberg from Regensburg University Hospital and L. Siam, M. Schaffrinski and D. Egert from Göttingen University Hospital, Germany, for their contribution in collecting and processing human samples. The study was supported by grants to N.E. from the Melanoma Research Alliance (award ID 826222), a Breakthrough Award from the US Department of Defense (BCRP award ID W81XWH2110394), Israel Science Foundation Personalized Medicine Program (IPMP no. 3495/19) and a research grant from the Tel Aviv University Cancer Biology Research Center. N.E. and T.P. were supported by a grant from the German Research Foundation (PU 355/4-1). T.P. was supported by SFB/TRR 305/1 (B03).
Author information
Authors and Affiliations
Contributions
O.A., Y.Z., H.D. and N.E. conceived of and designed this study. O.A., Y.Z., N.C., T.P. and N.E. developed the methods. O.A., Y.Z., R.B., L.M. and N.C. performed the experiments. G.G., Y.S., T.S., D.M. and L.M. performed formal analysis. A.A.K., S.H., V.Y., S.H., J.A.H., A.B., S.Y.K., L.N, I.B. and T.P. provided resources. O.A., Y.Z., N.C. and N.E. wrote the original draft. O.A., Y.Z., N.C. and N.E. revised and edited the manuscript. N.C. and N.E. administered this project. N.E. supervised the study. All authors discussed the results and provided feedback on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Frank Winkler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Proteomic analysis of secreted proteins in blood of mice with melanoma or breast cancer brain metastasis.
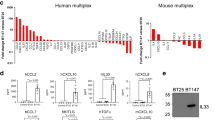
Heatmap showing fold change values of secreted proteins measured by Proteome profiler Mouse XL Cytokine Array. Mean grey value was quantified by ImageJ. Heatmap represents Log2 (BrM/Normal) values.
Extended Data Fig. 2 LCN2 expression is induced by tumor cell-secreted factors, and is not necessary for primary tumor growth.
a. Gating strategy for FACS isolation of different cell populations from primary tumors 2.5 weeks following BT-RMS or BT-EO771 orthotopic injection. b–d. qPCR analysis of Lcn2 from BMDM, dermal fibroblasts and C166 endothelial cells treated in vitro with BT-RMS CM (BT_RMS CM) versus serum free medium (SFM). 3 biological repeats, error bars represent mean ± SD (Student’s t-test, two-sided). e. qPCR analysis of Lcn2 in primary dermal fibroblasts treated with secreted factors from RMS and sBT-RMS, error bars represent mean ± SD (SFM n = 3, RMS CM n = 3, SBT CM n = 3 biological replicates) (one-way ANOVA). f. Primary tumor growth curve of WT and Lcn2−/− mice orthotopically injected with BT-RMS melanoma cells, measured manually by caliper, (WT n = 10, Lcn2−/− n = 8 mice) (Repeated measure ANOVA). g. Primary tumor weight at time of resection of WT and Lcn2−/− mice orthotopically injected with BT-EO771 cells, (WT n = 10, Lcn2−/− n = 10 mice), dots represent individual mice, error bars represent s.e.m. (Student’s t-test, two-sided).
Extended Data Fig. 3 Brain metastatic burden and survival in mice injected with breast cancer cells.
a. Experimental scheme analyzed in (b,c). b. Survival curve analysis of WT and Lcn2-/- mice injected intracardially with BT-EO771 cells, (WT n = 10, Lcn2-/- n = 9 mice) (Kaplan–Meier curve, log-rank test). c. Quantification of brain metastatic burden for mice in (a), quantified as % CD45- mCherry+ tumor cells/live cells, (ctrl n = 9, WT n = 9, Lcn2-/- n = 7 mice) (one-way ANOVA). d. Gating strategy for isolation by FACS of different cells populations from WT and Lcn2-/- BrM mice injected intracardially with BT-RMS or BT-EO771 cells 18 days after injection.
Extended Data Fig. 4 Astrocytes activate pro-inflammatory signaling in an LCN2-dependent manner, and SLC22A17 is required for astrocyte response to LCN2.
a. Expression of SLC22A17 in bulk RNA-seq of different cell populations isolated from samples of human gliomas (CD45- n = 23, MG n = 21, MDM n = 17, Neutrophils n = 16, T cells n = 22 patients) (one-way ANOVA), (Brain TIME dataset). b. LCN2 is sufficient to induce pro-inflammatory signaling in primary astrocytes. qPCR analysis of inflammatory gene signature in primary astrocytes incubated with 10ug/ml rLCN2. Error bars represent SD, dots represent two biological repeats with technical replicates (two-way ANOVA). c. qPCR analysis of inflammatory gene signature in primary astrocytes transfected with siRNA targeting Slc22a17 or with control siRNA (siSlc22a17 or siScramble). Astrocytes were then treated with 10ug/ml rLCN2 or SFM for 24 h. Error bars represent SD, 4 biological repeats in duplicates. d. LCN2 measured in CM of BT-RMS cells or granulocytes activated by BT-RMS CM, error bars represent SD, dots represent 3 biological repeats (two-way ANOVA). e, f. Activation of astrocytes was quantified by number of GFAP + cells/ field. Representative images are shown from n = 6,8,8 mice per group, error bars represent mean ± SD. 6 fields X 1 sections per mouse were analyzed (one-way ANOVA). g, h. Expression level of inflammatory gene signature measured by qPCR in RNA of FACS sorted astrocytes in vivo from WT or Lcn2-/- mice with BrM following BT-RMS or BT-EO771 injection. Dots represent individual mice, line indicates median, plot shows mean to max (melanoma: Ctrl n = 4, WT n = 7,10, Lcn2-/- n = 5,9 mice), (breast: Ctrl n = 6, WT n = 5, Lcn2-/- n = 5,7 mice) (two-way ANOVA). i. Expression level of inflammatory gene signature measured by qPCR of FACS sorted astrocytes in vivo from WT or Lcn2-/- normal mice, (WT n = 3, Lcn2-/- n = 2 mice). j, k. Expression level of immunosuppressive gene signature measured by qPCR of sorted MG/MDM in vivo from WT or Lcn2-/- mice, 3 weeks following BT-RMS or BT-EO771 intracardiac injection, line indicates median, plot shows mean to max (Melanoma WT n = 7, Lcn2-/- n = 5, Breast WT n = 5, Lcn2-/- n = 6 mice) (two-way ANOVA).
Extended Data Fig. 5 LCN2 induces recruitment of immune suppressive granulocytes to brain in the in the context of BrM.
a. Quantification of migrated Ly6G+ granulocytes or Ly6C+ Ly6G− monocytes toward normal astrocytes or astrocytes treated 24 h with 10ug/ml rLCN2. Dots represent individual wells, 4 biological repeats in duplicates, error bars represent mean ± SD (Student’s t-test, two-sided). b. Immune profiling of bone marrow cell populations by flow cytometry of 8-week-old C57BL/6 WT or Lcn2−/− mice, (WT n = 5, Lcn2−/− n = 5 mice). c. Immune profiling of CD11b+ myeloid cells by flow cytometry of normal WT or Lcn2−/− mice (male WT n = 5, Lcn2−/− n = 3, female WT n = 6, Lcn2−/− n = 3 mice). d,e. Expression level of immunosuppressive gene signature measured by qPCR of FACS sorted granulocytes in vivo from WT or Lcn2−/− mice, 3 weeks following BT-RMS intracardiac injection, line indicates median, plot shows mean to max (Melanoma WT n = 11, Lcn2−/− n = 9, Breast WT n = 5, Lcn2−/− n = 6 mice) (two-way ANOVA).
Extended Data Fig. 6 Bone marrow-derived granulocyte induce increasing systemic LCN2 levels in plasma and inflammatory activation of astrocytes.
a. qPCR analysis of Lcn2 expression in different cell populations sorted from bone marrow of normal C57BL/6 males: CD45-, CD45+CD11b+Ly6CinterLy6G+ granulocytes, CD45+CD11b+Ly6C+Ly6G- monocytes, CD45+CD11b-CD3+ B220- T cells, CD45+CD11b-CD3-B220+ B cells. Dots represent individual mice (n = 4 mice per group), line indicates median, whisker shows mean to max (one-way ANOVA). b. ‘LCN2 BM contribution’ calculated by expression of LCN2 in different cell populations from a, multiplied by their abundance in BM from Extended Data Fig. 5b. Results are presented as percent of total. c. LCN2 ELISA in blood, one and two weeks following BMT. Dots represent individual mice, error bars represent s.e.m., (WT n = 9, Lcn2-/- n = 9 mice) (one-way ANOVA). d. LCN2 levels in blood of mice at endpoint measured by ELISA, (WT n = 7, Lcn2-/- n = 7 mice), dots represent individual mice, error bars represent s.e.m. (Student’s t-test, two-sided). e. Expression level of inflammatory gene signature measured by qPCR in RNA of FACS sorted astrocytes in vivo from WT mice that underwent BMT as described in (Fig. 4a). Dots represent individual mice, line indicates median, plot shows mean to max (Ctrl n = 4, WT n = 6, Lcn2-/- n = 6 mice) (two-way ANOVA).
Extended Data Fig. 7 LCN2 is highly expressed in granulocytes in human BrM and can help direct patient’s care in combination with clinically used prognostic factors.
a. Immunofluorescence staining in frozen sections of resected human brain metastases from melanoma, breast and lung primary origin. Co-localization of LCN2 with CD66b (granulocytes). Representative images of separate staining are shown from n = 2 patients samples stained per cancer type. b. Five-year survival curve analysis of patients with low versus high LCN2 levels in patients with lung cancer BrM. The cutoff between high and low levels was defined as the median LCN2 level (Kaplan–Meier curve, log-rank test). c. Five-year survival curve analysis of patients with KPS score over or under 70, in patients with lung cancer BrM. d. 5-year survival curve analysis of patients with KPS score < 70, stratified to low versus high LCN2 levels in patients from Fig. 7h.
Supplementary information
Supplementary Table 1
Primers list.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data.
Source Data Fig. 1
Unprocessed cytokine array membrane.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adler, O., Zait, Y., Cohen, N. et al. Reciprocal interactions between innate immune cells and astrocytes facilitate neuroinflammation and brain metastasis via lipocalin-2. Nat Cancer 4, 401–418 (2023). https://doi.org/10.1038/s43018-023-00519-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-023-00519-w
This article is cited by
-
Biological profile of breast cancer brain metastasis
Acta Neuropathologica Communications (2025)
-
Pan-cancer drivers of metastasis
Molecular Cancer (2025)
-
Plasma metabolomic signatures for copy number variants and COVID-19 risk loci in Northern Finland populations
Scientific Reports (2025)
-
Neutrophils physically interact with tumor cells to form a signaling niche promoting breast cancer aggressiveness
Nature Cancer (2025)
-
A new, immunocompetent brain-metastatic mouse model of HER2-positive breast cancer
Clinical & Experimental Metastasis (2025)