Abstract
The study of cardiac physiology is hindered by physiological differences between humans and small-animal models. Here we report the generation of multi-chambered self-paced vascularized human cardiac organoids formed under anisotropic stress and their applicability to the study of cardiac arrhythmia. Sensors embedded in the cardiac organoids enabled the simultaneous measurement of oxygen uptake, extracellular field potentials and cardiac contraction at resolutions higher than 10 Hz. This microphysiological system revealed 1 Hz cardiac respiratory cycles that are coupled to the electrical rather than the mechanical activity of cardiomyocytes. This electro-mitochondrial coupling was driven by mitochondrial calcium oscillations driving respiration cycles. Pharmaceutical or genetic inhibition of this coupling results in arrhythmogenic behaviour. We show that the chemotherapeutic mitoxantrone induces arrhythmia through disruption of this pathway, a process that can be partially reversed by the co-administration of metformin. Our microphysiological cardiac systems may further facilitate the study of the mitochondrial dynamics of cardiac rhythms and advance our understanding of human cardiac physiology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Sequencing data are available at NCBI GEO, under the accession number GSE234907. Data for the figures are provided with this paper. All data supporting the results of this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
Code availability
The custom analysis software is available at https://github.com/mohammadghosheh95/Heart-on-a-Chip.
References
Benjamin, E. J. et al. Heart disease and stroke statistics–2017 update: a report from the American Heart Association. Circulation 135, e146–e603 (2017).
Benjamin, E. J. et al. Heart disease and stroke statistics–2019 update: a report from the American Heart Association. Circulation 139, e56–e528 (2019).
Fryar, C. D., Chen, T. C. & Li, X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Brief 103, 1–8 (2012).
Huch, M., Knoblich, J. A., Lutolf, M. P. & Martinez-Arias, A. The hope and the hype of organoid research. Development 144, 938–941 (2017).
Bertero, E. & Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 15, 457–470 (2018).
Rosano, G. M. & Vitale, C. Metabolic modulation of cardiac metabolism in heart failure. Card. Fail. Rev. 4, 99–103 (2018).
Doehner, W., Frenneaux, M. & Anker, S. D. Metabolic impairment in heart failure: the myocardial and systemic perspective. J. Am. Coll. Cardiol. 64, 1388–1400 (2014).
von Haehling, S., Doehner, W. & Anker, S. D. Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc. Res. 73, 298–309 (2007).
Bonnet, D. et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation 100, 2248–2253 (1999).
Gheorghiade, M., Colucci, W. S. & Swedberg, K. Beta-blockers in chronic heart failure. Circulation 107, 1570–1575 (2003).
Hamer, A. W., Arkles, L. B. & Johns, J. A. Beneficial effects of low dose amiodarone in patients with congestive cardiac failure: a placebo-controlled trial. J. Am. Coll. Cardiol. 14, 1768–1774 (1989).
Kloner, R. A., Shi, J. & Dai, W. New therapies for reducing post-myocardial left ventricular remodeling. Ann. Transl. Med. 3, 20 (2015).
Brown, D. A. et al. Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 14, 238–250 (2017).
Aon, M. A., Cortassa, S., Marbán, E. & O’Rourke, B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 278, 44735–44744 (2003).
Romashko, D. N., Marban, E. & O’Rourke, B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc. Natl Acad. Sci. USA 95, 1618–1623 (1998).
Bers, D. M. Cardiac excitation–contraction coupling. Nature 415, 198–205 (2002).
Gottlieb, R. A. & Bernstein, D. Mitochondria shape cardiac metabolism. Science 350, 1162–1163 (2015).
Wang, S. C. et al. Pathological basis of cardiac arrhythmias: vicious cycle of immune-metabolic dysregulation. Cardiovasc. Disord. Med. https://doi.org/10.15761/CDM.1000158 (2018).
Edwards, A. G. & Louch, W. E. Species-dependent mechanisms of cardiac arrhythmia: a cellular focus. Clin. Med. Insights Cardiol. 11, 1179546816686061 (2017).
Milani-Nejad, N. & Janssen, P. M. L. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol. Ther. 141, 235–249 (2014).
Ebert, A. et al. Proteasome-dependent regulation of distinct metabolic states during long-term culture of human iPSC-derived cardiomyocytes. Circ. Res. 125, 90–103 (2019).
Ronaldson-Bouchard, K. et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239–243 (2018).
Giacomelli, E. et al. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development 144, 1008–1017 (2017).
Noor, N. et al. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 6, 1900344 (2019).
Skylar-Scott, M. A. et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 5, eaaw2459 (2019).
Mills, R. J. et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc. Natl Acad. Sci. USA 114, E8372–e8381 (2017).
Mills, R. J. et al. Drug screening in human PSC-cardiac organoids identifies pro-proliferative compounds acting via the mevalonate pathway. Cell Stem Cell 24, 895–907.e6 (2019).
Richards, D. J. et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 4, 446–462 (2020).
Li, R. A. et al. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials 163, 116–127 (2018).
Goldfracht, I. et al. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 11, 75 (2020).
Zhao, Y. et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 176, 913–927.e8 (2019).
Lind, J. U. et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 16, 303–308 (2017).
Lind, J. U. et al. Cardiac microphysiological devices with flexible thin-film sensors for higher-throughput drug screening. Lab Chip 17, 3692–3703 (2017).
Oleaga, C. et al. Long-term electrical and mechanical function monitoring of a human-on-a-chip system. Adv. Funct. Mater. 29, 1805792 (2019).
Maoz, B. M. et al. Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip 17, 2294–2302 (2017).
Tian, B. et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 11, 986–994 (2012).
Feiner, R. et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 15, 679–685 (2016).
Drakhlis, L. et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 39, 737–746 (2021).
Miquerol, L. & Kelly, R. G. Organogenesis of the vertebrate heart. Wiley Interdiscip. Rev. Dev. Biol. 2, 17–29 (2013).
Moorman, A., Webb, S., Brown, N. A., Lamers, W. & Anderson, R. H. Development of the heart: (1) formation of the cardiac chambers and arterial trunks. Heart 89, 806–814 (2003).
Lin, C. J., Lin, C. Y., Chen, C. H., Zhou, B. & Chang, C. P. Partitioning the heart: mechanisms of cardiac septation and valve development. Development 139, 3277–3299 (2012).
Swift, J. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Gordan, R., Gwathmey, J. K. & Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 7, 204–214 (2015).
Mackin, C. et al. Intravenous amiodarone and sotalol impair contractility and cardiac output, but procainamide does not: a Langendorff study. J. Cardiovasc. Pharmacol. Ther. 24, 288–297 (2019).
Karbassi, E. et al. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 17, 341–359 (2020).
Wegner, A., Meiser, J., Weindl, D. & Hiller, K. How metabolites modulate metabolic flux. Curr. Opin. Biotechnol. 34, 16–22 (2015).
Collins-Nakai, R. L., Noseworthy, D. & Lopaschuk, G. D. Epinephrine increases ATP production in hearts by preferentially increasing glucose metabolism. Am. J. Physiol. 267, H1862–H1871 (1994).
Itzhaki, I. et al. Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J. Am. Coll. Cardiol. 60, 990–1000 (2012).
Lee, C. H. & Ruben, P. C. Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels 2, 407–412 (2008).
Farman, G. P. et al. Blebbistatin: use as inhibitor of muscle contraction. Pflugers Arch. 455, 995–1005 (2008).
Taxin, Z. H., Neymotin, S. A., Mohan, A., Lipton, P. & Lytton, W. W. Modeling molecular pathways of neuronal ischemia. Prog. Mol. Biol. Transl. Sci. 123, 249–275 (2014).
Fonteriz, R. I. et al. Monitoring mitochondrial [Ca(2+)] dynamics with rhod-2, ratiometric pericam and aequorin. Cell Calcium 48, 61–69 (2010).
Tsien, R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature 290, 527–528 (1981).
Berry, W., Dakhil, S., Modiano, M., Gregurich, M. & Asmar, L. Phase III study of mitoxantrone plus low dose prednisone versus low dose prednisone alone in patients with asymptomatic hormone refractory prostate cancer. J. Urol. 168, 2439–2443 (2002).
Evison, B. J., Sleebs, B. E., Watson, K. G., Phillips, D. R. & Cutts, S. M. Mitoxantrone, more than just another topoisomerase II poison. Med. Res. Rev. 36, 248–299 (2016).
Scott, L. J. & Figgitt, D. P. Mitoxantrone: a review of its use in multiple sclerosis. CNS Drugs 18, 379–396 (2004).
Ewer, M. S. & Ewer, S. M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 12, 547–558 (2015).
Romitan, D. M. et al. Cardiomyopathies and arrhythmias induced by cancer therapies. Biomedicines https://doi.org/10.3390/biomedicines8110496 (2020).
Guglin, M., Aljayeh, M., Saiyad, S., Ali, R. & Curtis, A. B. Introducing a new entity: chemotherapy-induced arrhythmia. Europace 11, 1579–1586 (2009).
Neri, B., Cini-Neri, G. & D’Alterio, M. Effect of anthracyclines and mitoxantrone on oxygen uptake and ATP intracellular concentration in rat heart slices. Biochem. Biophys. Res. Commun. 125, 954–960 (1984).
Arduino, D. M. et al. Systematic identification of MCU modulators by orthogonal interspecies chemical screening. Mol. Cell 67, 711–723.e7 (2017).
De Mario, A. et al. Identification and functional validation of FDA-approved positive and negative modulators of the mitochondrial calcium uniporter. Cell Rep. 35, 109275 (2021).
Loubiere, C. et al. The energy disruptor metformin targets mitochondrial integrity via modification of calcium flux in cancer cells. Sci. Rep. 7, 5040 (2017).
Zhao, H. et al. AMPK-mediated activation of MCU stimulates mitochondrial Ca(2+) entry to promote mitotic progression. Nat. Cell Biol. 21, 476–486 (2019).
Moscovitz, H. L., Donoso, E., Gelb, I. J. & Welkowitz, W. Intracardiac phonocardiography: correlation of mechanical, acoustic and electric events of the cardiac cycle. Circulation 18, 983–988 (1958).
Morad, M. & Orkand, R. K. Excitation-concentration coupling in frog ventricle: evidence from voltage clamp studies. J. Physiol. 219, 167–189 (1971).
Wendt-Gallitelli, M. F. & Isenberg, G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J. Physiol. 435, 349–372 (1991).
Lee, K. S. A new technique for the simultaneous recording of oxygen consumption and contraction of muscle: the effect of ouabain on cat papillary muscle. J. Pharmacol. Exp. Ther. 109, 304–312 (1953).
Lentini, E. A. Myocardial oxygen consumption as influenced by isotonic contractions. Proc. Soc. Exp. Biol. Med. 109, 869–872 (1962).
Isenberg, G., Han, S., Schiefer, A. & Wendt-Gallitelli, M. F. Changes in mitochondrial calcium concentration during the cardiac contraction cycle. Cardiovasc. Res. 27, 1800–1809 (1993).
Reiermann, H. J., Herzig, J. W. & Rüegg, J. C. Ca++ activation of ATPase activity, ATP-Pi exchange, and tension in briefly glycerinated heart muscle. Basic Res. Cardiol. 72, 133–139 (1977).
O’Rourke, B., Ramza, B. M. & Marban, E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science 265, 962–966 (1994).
Robert, V. et al. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. EMBO J. 20, 4998–5007 (2001).
Wescott, A. P., Kao, J. P. Y., Lederer, W. J. & Boyman, L. Voltage-energized calcium-sensitive ATP production by mitochondria. Nat. Metab. 1, 975–984 (2019).
Patterson, A. J. & Zhang, L. Hypoxia and fetal heart development. Curr. Mol. Med. 10, 653–666 (2010).
Bakkehaug, J. P. et al. Myosin activator omecamtiv mecarbil increases myocardial oxygen consumption and impairs cardiac efficiency mediated by resting myosin ATPase activity. Circ. Heart Fail. 8, 766–775 (2015).
Lewis, G. D. et al. Effect of omecamtiv mecarbil on exercise capacity in chronic heart failure with reduced ejection fraction: the METEORIC-HF randomized clinical trial. JAMA 328, 259–269 (2022).
Teerlink, J. R. et al. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet 388, 2895–2903 (2016).
Bick, A. G. et al. Cardiovascular homeostasis dependence on MICU2, a regulatory subunit of the mitochondrial calcium uniporter. Proc. Natl Acad. Sci. USA 114, E9096–e9104 (2017).
Pan, X. et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 15, 1464–1472 (2013).
Lambert, J. P. et al. MCUB regulates the molecular composition of the mitochondrial calcium uniporter channel to limit mitochondrial calcium overload during stress. Circulation 140, 1720–1733 (2019).
Wu, Y. et al. The mitochondrial uniporter controls fight or flight heart rate increases. Nat. Commun. 6, 6081 (2015).
Yellon, D. M. & Hausenloy, D. J. Myocardial reperfusion injury. N. Engl. J. Med. 357, 1121–1135 (2007).
Kwong, J. C. et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N. Engl. J. Med. 378, 345–353 (2018).
Tiburcy, M. et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 135, 1832–1847 (2017).
Fleischer, V. et al. Cardiotoxicity of mitoxantrone treatment in a German cohort of 639 multiple sclerosis patients. J. Clin. Neurol. 10, 289–295 (2014).
Xie, A. et al. Mitochondrial Ca(2+) influx contributes to arrhythmic risk in nonischemic cardiomyopathy. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.117.007805 (2018).
Schweitzer, M. K. et al. Suppression of arrhythmia by enhancing mitochondrial Ca(2+) uptake in catecholaminergic ventricular tachycardia models. JACC Basic Transl. Sci. 2, 737–747 (2017).
Burridge, P. W. et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 11, 855–860 (2014).
Pires, R. H., Shree, N., Manu, E., Guzniczak, E. & Otto, O. Cardiomyocyte mechanodynamics under conditions of actin remodelling. Phil. Trans. R. Soc. Lond. B 374, 20190081 (2019).
Li, Z., Guo, X., Palmer, A. F., Das, H. & Guan, J. High-efficiency matrix modulus-induced cardiac differentiation of human mesenchymal stem cells inside a thermosensitive hydrogel. Acta Biomater. 8, 3586–3595 (2012).
Liu, M., Sun, J., Sun, Y., Bock, C. & Chen, Q. Thickness-dependent mechanical properties of polydimethylsiloxane membranes. J. Micromech. Microeng. 19, 035028 (2009).
Soofi, S. S., Last, J. A., Liliensiek, S. J., Nealey, P. F. & Murphy, C. J. The elastic modulus of Matrigel as determined by atomic force microscopy. J. Struct. Biol. 167, 216–219 (2009).
Ebrahimi, A. P. Mechanical properties of normal and diseased cerebrovascular system. J. Vasc. Interv. Neurol. 2, 155–162 (2009).
Dutta, D. et al. Non-invasive assessment of elastic modulus of arterial constructs during cell culture using ultrasound elasticity imaging. Ultrasound Med. Biol. 39, 2103–2115 (2013).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Mi, H., Muruganujan, A., Ebert, D., Huang, X. & Thomas, P. D. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 47, D419–D426 (2018).
Xia, J., Gill, E. E. & Hancock, R. E. W. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat. Protoc. 10, 823–844 (2015).
Ehrlich, A. et al. Microphysiological flux balance platform unravels the dynamics of drug induced steatosis. Lab Chip 18, 2510–2522 (2018).
Joung, J. et al. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat. Protoc. 12, 828–863 (2017).
Ou, Q. et al. Physiological biomimetic culture system for pig and human heart slices. Circ. Res. 125, 628–642 (2019).
Ludikhuize, M. C., Meerlo, M., Burgering, B. M. T. & Rodríguez Colman, M. J. Protocol to profile the bioenergetics of organoids using Seahorse. STAR Protoc. 2, 100386 (2021).
Sigg, C. & Buhmann, J. Expectation-maximization for sparse and non-negative PCA. In Proc. 25th International Conference on Machine Learning 960–967 (Association for Computing Machinery, 2008).
Wickham, H. & Sievert, C. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Acknowledgements
Funding was provided by the European Research Council Consolidator Grant OCLD (project no. 681870) and generous gifts from the Nikoh Foundation and the Sam and Rina Frankel Foundation. M.G. was supported by a Neubauer Foundation Graduate Fellowship. We thank O. Leitersdorf, Y. Kroiz, J. Gotlib, D. Viner, I. Shweky, H. Naimi, B. A. Berke, M. Ehrlich and S. Regenbaum. Figure 8a was generated using BioRender.com.
Author information
Authors and Affiliations
Contributions
M.G., A.E. and Y.N. conceived the hypothesis. M.G., A.E., M.A., L.G. and Y.N. designed the experiment. M.G., A.E., M.A., K.I., M.C., A.F. and Y.N. performed the experiments. Y.M. provided the porcine heart and prepared the ex-vivo heart tissue. M.G. and A.E. analysed the results. M.G., A.E. and Y.M. wrote the manuscript. M.G., A.E. and I.G. built the system. M.C., L.G. and Y.N supervised the project. All authors read the manuscript and agree with its contents.
Corresponding author
Ethics declarations
Competing interests
Y.N. and A.E. are employees of Tissue Dynamics. M.G., A.E. and Y.N. filed a patent application through Hebrew University (US202163242091P; 2019, Israel). The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Alessandro Prigione and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Vascular network development during cardiac organoid formation.
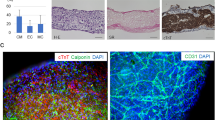
a, Representative time-lapse sequence confocal images depicting the formation of vascular networks in cardiac organoids formed from UN-1, ACS-1021, and ACS-1028 hiPSC-derived cardiomyocytes. Confocal microscopy shows the distribution of GFP-expressing rat microvascular cardiac endothelial cells (CECs) in the organoid. Vascular networks are apparent by day 10, stabilizing into a capillary-like network, distributed within the organoid at 25 d. UN-1 cardiac organoid images were taken from Fig.1b. Scale bar, 100 μm. b, Relative gene expression of the rat microvascular cardiac endothelial cells (CECs) and isolated rat primary endothelial cells. The CECs are showing expression signatures comparable with those of isolated rat primary endothelial cells across multiple endothelial markers. Anti-phospho-Nuclear Receptor (NR4A1), Cadherin 5 (CDH5), Von Willebrand factor (VWF), and tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (TIE1) are similarly expressed among the CECs used and isolated rat primary endothelial cells.
Extended Data Fig. 2 Functional comparison of hiPSC-CMs and human cardiac organoids.
a, Principal component analysis (PCA) of 513 genes differentially expressed between hiPS-derived cardiomyocytes and vascularized cardiac organoids (methods). Cardiac organoids cluster with adult, but not fetal cardiomyocytes. b, Principal component analysis (PCA) of 513 genes differentially expressed between hiPS-derived cardiomyocytes and vascularized cardiac organoids (methods) separated into PC components. PC1 gene set is enriched for angiogenesis and cell adhesion, clustering the non-beating AC16 cell line, the negative control, with the mature heart tissue. This clearly suggests that PC1 is less relevant for comparative analysis. c, Visual contraction analysis of UN-1 vascularized cardiac organoids treated with DMSO (Control), 10 µM amiodarone, or 100 µM epinephrine (Supplementary. Video 3), normalized to the highest and lowest signal recorded through the entire measurement duration (30 seconds; methods). Analysis shows that untreated organoids acquire a homogenous synchronized spontaneous beating of 66 ± 5 beats per minute. Stimulation with 100 µM epinephrine increases the contraction rate to 88 ± 7 bpm and relative contraction by 18% (n = 5, p < 0.001), while stimulation with 10 µM amiodarone decreased the rate to 52 ± 4 bpm and contraction by 28% (n = 5, p < 0.001), resulting in a physiological-like response to the drugs. Mean of 5 biological replicates; error bars, s.e.m. Significance was determined using a one-way ANOVA with Dunnett correction. Graphs were taken from Fig. 3d. d, Visual contraction analysis of UN-1 hiPSC-derived cardiomyocytes (hiPSC-CMs) treated with DMSO (Control), 10 µM amiodarone, or 100 µM epinephrine. Analysis shows that untreated hiPSC-CMs acquire a homogenous unsynchronized beating of 97 ± 4 beats per minute. Stimulation with 100 µM epinephrine increases the contraction rate to 106 ± 4 bpm and relative contraction by 18% (n = 3, p < 0.01), while stimulation with 10 µM amiodarone decreased the rate to 92 ± 3 bpm and contraction by 28% (n = 3, p < 0.01). Mean of 3 biological replicates; error bars, s.e.m. Significance was determined using a one-way ANOVA with Dunnett correction. e, Seahorse MitoStress assay nested analysis of UN-1 cardiac organoids, UN-1 hiPSC-derived cardiomyocytes (hiPSC-CMs), and cardiac endothelial cells (CECs). CECs show basal respiration equal to 4.5% of the basal respiration of the cardiac organoids and less than 2% of maximal respiration, indicating that changes in respiration are attributed to changes in the cardiomyocytes (n = 9, p < 0.001). Significance was determined using one-way ANOVA and Dunnett multiple comparison correction. Lines represent independent experiments, Error bars mark standard error of mean among n = 3 biological repeats.
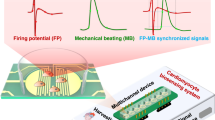
Extended Data Fig. 3 Establishment of an Integrated 2-PMT Heart-on-a-chip platform.
a, Scheme and typical measurements depicting the advantages of using a 2-PMT system over a single PMT system. The addition of the second detector (cPMT), which measures the excitation signal, reduces the noise and enables accurate measurements at sub-second resolution. The second PMT also allows an emission-independent measurement of tissue contraction (methods). b, Representative calibration measurements of the reflected signal measured by the second PMT in different displacements. Curve fitting displays a sigmoidal relationship between the emission intensity, measured by peak-to-peak voltage (VP-P), and the sensor displacement. Cardiac displacement was measured by the embedded oxygen beads inside the cardiac organoids during a contraction when the beads move at different distances from the focal point. The Sigmoidal fit shows a correlation of R-square: 0.9835 and RMSE below 4. c, Representative continuous interstitial oxygen measurements in cardiac organoids measured continuously over 10 hours. Measurements show steady recordings that are unaffected by photobleaching or system sensitivity loss at prolonged measurements. Oxygen content and oscillation were unchanged even after 10 hours of measurement.
Extended Data Fig. 4 Quantitative and dynamic analyses of contraction, field potential, and oxygen content in vascularized cardiac organoids.
a, Representative simultaneous measurements and (b) Fast Fourier Transformation (FFT) analysis of contraction, field potential, and interstitial oxygen during spontaneous beating of cardiac organoids formed from UN-1, ACS-1021, and ACS-1028 hiPSC-derived cardiomyocytes. Interstitial oxygen concentration shows oscillatory behaviour during the cardiac cycle, yielding distinct single-frequency peaks in FFT analysis correlated to the mechanical and electrical behaviour of the cardiac tissue. UN-1 cardiac organoid graphs were taken from Fig. 4f–h. Nested analysis of the organoid’s contraction and oxygen oscillation (c) frequency and (d) amplitude in UN-1, ACS-1021, and ACS-1028 cardiac organoids. Treatment with 10 μM of myosin II inhibitor Blebbistatin. Blebbistatin completely inhibits the contraction of vascularized cardiac organoids (n = 3, p < 0.001) but does not affect the field potential or oxygen oscillation (n = 3, p > 0.05). Treatment with 25 μM of Nav channel inhibitor Tetrodotoxin (TTX) resulted in a complete loss of field potential generation, the coupled mechanical contraction (n = 3, p < 0.001), and a concurrent loss of oxygen oscillations (n = 3, p < 0.001). Middle represents mean of 3 biological repeats for each line; error bars,s.e.m. Significance was determined using a two-tailed nested t-test. e, Representative graph of the interstitial oxygen behaviours in ACS-1021 cardiac organoids following treatment with 25 μM of Nav channel inhibitor Tetrodotoxin (TTX). After 7 minutes of exposure to TTX, a complete loss of field potential generation occurs, coupled with decay in oxygen oscillations (n = 3). Graph was taken from Fig. 4l. Middle represents mean of 3 biological repeats for each line; error bars,s.e.m. Significance was determined using a two-tailed nested t-test.
Extended Data Fig. 5 Real-time metabolic measurement of vascularized cardiac organoids under epinephrine stimulation.
Nested analysis of the organoid’s contraction and oxygen oscillation (a) frequency and (b) amplitude during prolonged stimulation by 100 µM epinephrine in UN-1, ACS-1021, and ACS-1028 cardiac organoids. Analysis shows that epinephrine exposure significantly increases the frequency of cardiac contraction and oxygen oscillations (n = 3, p < 0.001), and the amplitude of cardiac contraction and oxygen oscillation amplitudes (n = 3, p < 0.001). c, Analysis of the kinetic behaviour of the organoid’s contraction rate during prolonged stimulation by 100 µM epinephrine. Kinetic analysis suggests that epinephrine stimulation results in a sigmoidal-like change in the organoid contraction rate. Representative relation graphs between (d) contraction amplitude (contractility) to contraction rate, and (e) interstitial oxygen content to contraction rate during prolonged epinephrine stimulation. Analysis suggests a correlation between an increase in cardiac organoid contractility and oxygen consumption. f, Representative frequency histograms of interstitial oxygen measurements at 0, 15, and 90 minutes after stimulation with 100 µM epinephrine. Analysis shows that an increase in oxygen consumption correlates to an increase in interstitial oxygen content variability correlative to the increased oxygen amplitudes measured. Representative correlation analysis between (g) oxygen oscillation frequency to contraction frequency and (h) oxygen oscillation amplitude to contractility reveals a direct linear correlation between the oscillatory behaviour of the interstitial oxygen and organoid contraction. i, Representative graphs of the cardiac organoid’s contraction and interstitial oxygen content 60 mins prior to epinephrine stimulation and 5 hours post-stimulation with 100 µM epinephrine. Oxygen returns to the baseline value after 300 minutes, indicating hypoxia, stimulation-induced or ischemia-like injuries did not occur. Middle represents mean of 3 biological repeats for each line; error bars,s.e.m. Significance was determined using a two-tailed nested t-test.
Extended Data Fig. 6 Live mitochondrial imaging reveals oscillations in mitochondrial membrane potential.
a, Immunofluorescent micrograph of mitochondrial membrane potential measured using live imaging of TMRE dye (Supplementary. Video 2; methods). b, Kinetic analysis of mitochondrial membrane potential using TMRE. The mitochondrial membrane potential of hiPSC-derived cardiomyocytes oscillates in the frequency of contraction. The mitochondrial membrane potential of non-beating cells did not oscillate and was lower overall. Rainbow heatmaps of (c) mean mitochondrial membrane potential and (d) major oscillating frequency of (MMP) measured using TMRE (methods). The heatmaps suggest a correlation between areas with high mean mitochondrial membrane potential and oscillation frequency. e, Immunofluorescent micrograph of mitochondrial membrane potential measured using live imaging of JC-1 dye (Supplementary. Video 3; methods). f, Kinetic analysis of mitochondrial membrane potential following aggregation of JC-1 dye. Mitochondrial membrane potential showed distinct polarization peaks in contracting cells, while non-beating cells did not oscillate and show lower mitochondrial membrane potential overall. Rainbow heatmaps of (g) mean mitochondrial membrane potential and (h) major oscillating frequency of (MMP) measured using JC-1 (methods). Similar to the behaviour measured by TMRE, JC-1 heatmaps suggest a correlation between areas with high mean mitochondrial membrane potential and oscillation frequency. Scale bar, 25 μm.
Extended Data Fig. 7 CRISPR/Cas9 knockout of MCU disrupts electro-mitochondrial coupling and induces arrhythmic behaviour.
a, Kinetic measurements of mitochondrial calcium in beating 2D-cultured hiPSC-derived cardiomyocytes. Non-targeting sgRNA had no effect on [Ca2+]m showing a dominant frequency of 0.8 Hz, while MCU knockout (MCUKO) showed a 35–50% decrease in [Ca2+]m and oscillation magnitude while increasing oscillation rate to 1.3–1.4 Hz. b, Immunofluorescent confocal microscopy demonstrated a marked reduction in MCU expression in the MCU knockout (MCUKO) compared to the non-targeting control. Scale bar, 100 µm. c, Relative gene expression of the MCU gene was markedly reduced by 46% (n = 3, p < 0.001) and 27% (n = 3, p < 0.1) in the MCU knockout MCUKO cardiac organoids compared to the non-targeting cardiac organoids in ACS-1021 and UN-1 cell lines respectively. Mean of 3 biological replicates; error bars, s.e.m. d, Interstitial oxygen showed a 78% (n = 3, p < 0.001) and 126% (n = 4, p < 0.001) increase in the MCUKO cardiac organoids compared to the non-targeting organoids in ACS-1021 and UN-1 cell lines, respectively. Mean of 3 biological replicates; error bars,s.e.m. Significance was determined using a one-way ANOVA with Dunnett correction. UN-1 cardiac organoid graphs were taken from Fig. 6a.
Extended Data Fig. 8 Reduced oxygen consumption coordinated with electro-mitochondrial decoupling.
a, Representative kinetic measurements of interstitial oxygen content and mean oxygen content analysis in non-homogenous MCUKO or Non-targeting sgRNA cardiac organoids. Analysis shows a decrease in oscillation amplitude and an increase in oscillation frequency in the MCUKO organoids, coupled with an increase in mean oxygen content (n = 3, p < 0.001). Mean of 3 biological replicates; error bars,s.e.m. Significance was determined using two tailed t-test. Cardiac organoid graphs were taken from Fig. 6c. b, Representative kinetic measurements of interstitial oxygen content and mean oxygen content analysis in cardiac organoids treated with DMSO (Control), 10 μM mitoxantrone (Mitoxantrone) or 10 μM mitoxantrone, and 100 μM AMP-activated protein kinase (AMPK) activator metformin (Mitoxantrone + Metformin). Analysis shows a decrease in oscillation amplitude and an increase in oscillation frequency in the mitoxantrone-treated organoids, coupled with an increase in mean oxygen content (n = 3, p < 0.001). Metformin reverts these changes, restoring mean oxygen content to levels not significantly different than the control (n = 3, p > 0.05). Cardiac organoid graphs were taken from Fig. 6e. Mean of 3 biological replicates; error bars,s.e.m. Significance was determined using two tailed t-test. c, Representative kinetic measurements of interstitial oxygen content and mean oxygen content analysis in porcine cardiac tissue exposed to DMSO (control), 10 μM of blebbistatin, 10 µM mitoxantrone, or 10 µM mitoxantrone, and 100 μM metformin (mitoxantrone + metformin). Analysis shows that blebbistatin does not change oscillation amplitude and oscillation frequency or mean oxygen content (n = 3, p > 0.05). Mitoxantrone-treated organoids show a decrease in oscillation amplitude and an increase in oscillation frequency, coupled with an increase in mean oxygen content (n = 3, p < 0.001). Metformin partly reverts these changes, increasing mean oxygen content by 29% (n = 3, p < 0.001). Porcine cardiac tissue graphs were taken from Fig. 8d. Mean of 3 biological replicates; error bars,s.e.m. Significance was determined using one-way ANOVA with Dunnett multiple comparison correction.
Supplementary information
Supplementary Information
Supplementary figures.
Supplementary Video 1
Representative time-lapse sequence of brightfield images showing vascularized cardiac organoids during organoid formation.
Supplementary Video 2
Synchronized beating cardiac organoid embedded with oxygen sensors.
Supplementary Video 3
The effect of pharmaceutical stimulation of epinephrine and amiodarone on the contraction rate and contractility of a cardiac organoid.
Supplementary Video 4
Immunofluorescent live imaging of mitochondrial membrane-potential oscillations by TMRE stain in homogeneous beating hiPSC-derived cardiomyocytes.
Supplementary Video 5
Beating cardiac organoids under epinephrine stimulation at different timepoints.
Supplementary Video 6
MATLAB projection of the desynchronization of cardiac organoids due to MCU inhibition by KB-R7943.
Supplementary Video 7
Beating cardiac organoids embedded with oxygen sensors on an MEA chip.
Supplementary Video 8
Non-targeting CRISPR knockout cardiomyocyte wells display cardiac contractions, whereas none of the MCU CRISPR KO wells display cardiac contraction.
Supplementary Video 9
Time-lapse sequence of brightfield and fluorescent images of cardiac organoids loaded with the cell-impermeable Dextran-Texas Red.
Source data
Source Data for Figs. 3,5,6–8 and Extended Data Figs. 1–8
Source data and statistics.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosheh, M., Ehrlich, A., Ioannidis, K. et al. Electro-metabolic coupling in multi-chambered vascularized human cardiac organoids. Nat. Biomed. Eng 7, 1493–1513 (2023). https://doi.org/10.1038/s41551-023-01071-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-023-01071-9
This article is cited by
-
Advances in cardiac organoid research: implications for cardiovascular disease treatment
Cardiovascular Diabetology (2025)
-
Metamaterial-based injection molding for the cost-effective production of whole cuts
Nature Communications (2024)
-
Modeling acute myocardial infarction and cardiac fibrosis using human induced pluripotent stem cell-derived multi-cellular heart organoids
Cell Death & Disease (2024)
-
Improving human cardiac organoid design using transcriptomics
Scientific Reports (2024)