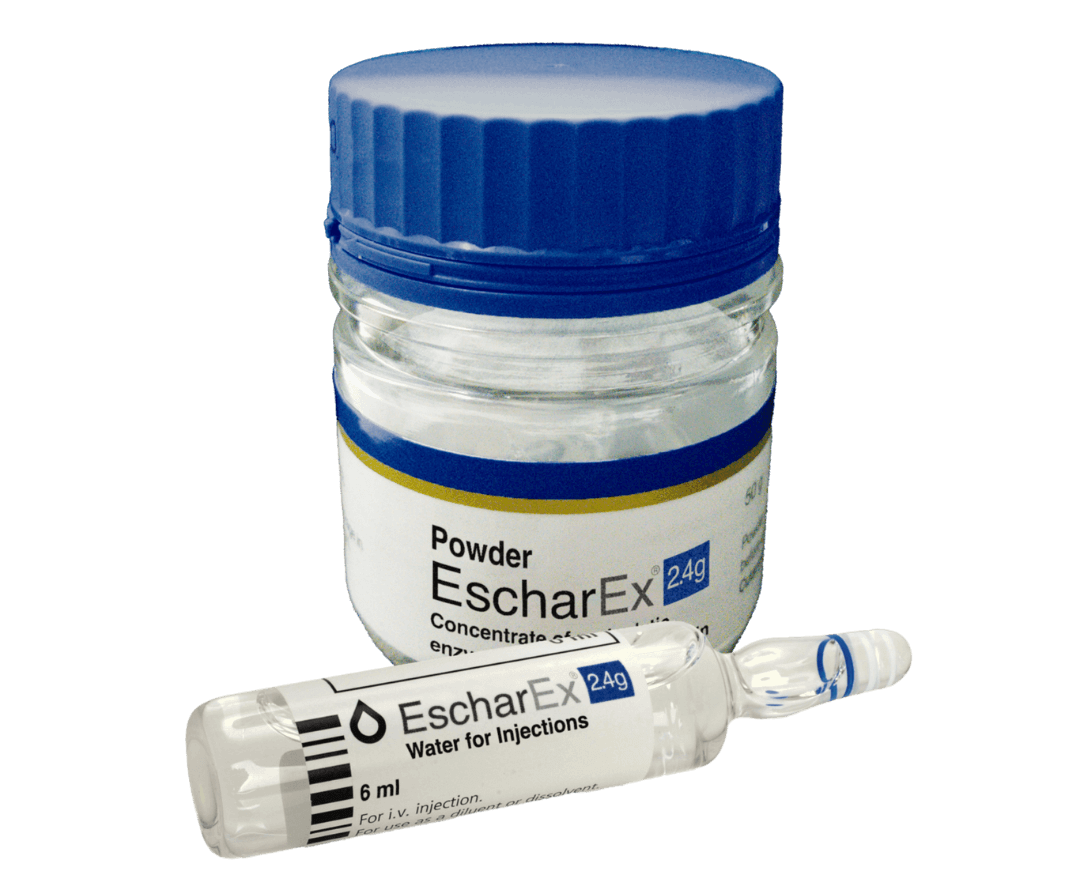
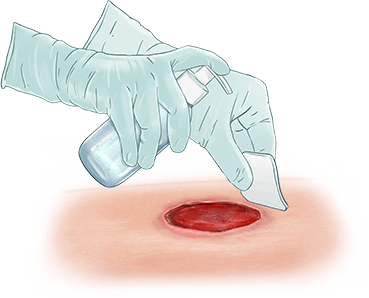
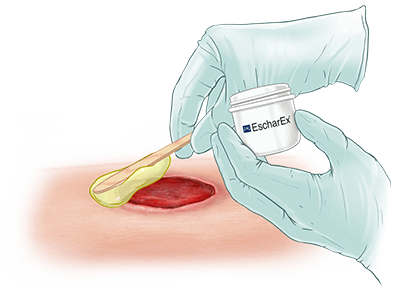
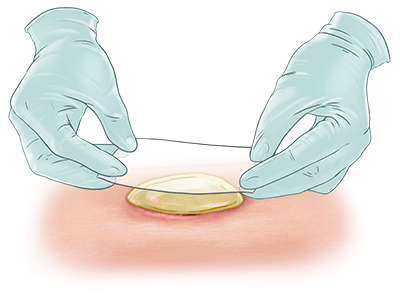
EscharEx® is a topical, enzymatic therapy in late-stage development for the debridement of chronic and hard-to-heal wounds. Designed for easy daily application, it offers a non-surgical approach to wound bed preparation by removing non-viable tissue, reducing bioburden, and promoting healing. It is currently being evaluated in a global Phase III study for venous leg ulcers, with additional studies planned for diabetic foot ulcers.
EscharEx® development is being supported in part by the European Innovation Council (EIC) through its accelerator program for the indication of diabetic foot ulcers.