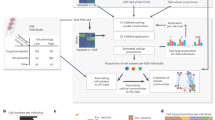
Abstract
Alzheimer’s disease (AD) has recently been associated with diverse cell states1,2,3,4,5,6,7,8,9,10,11, yet when and how these states affect the onset of AD remains unclear. Here we used a data-driven approach to reconstruct the dynamics of the brain’s cellular environment and identified a trajectory leading to AD that is distinct from other ageing-related effects. First, we built a comprehensive cell atlas of the aged prefrontal cortex from 1.65 million single-nucleus RNA-sequencing profiles sampled from 437 older individuals, and identified specific glial and neuronal subpopulations associated with AD-related traits. Causal modelling then prioritized two distinct lipid-associated microglial subpopulations—one drives amyloid-β proteinopathy while the other mediates the effect of amyloid-β on tau proteinopathy—as well as an astrocyte subpopulation that mediates the effect of tau on cognitive decline. To model the dynamics of cellular environments, we devised the BEYOND methodology, which identified two distinct trajectories of brain ageing, each defined by coordinated progressive changes in certain cellular communities that lead to (1) AD dementia or (2) alternative brain ageing. Thus, we provide a cellular foundation for a new perspective on AD pathophysiology that informs personalized therapeutic development, targeting different cellular communities for individuals on the path to AD or to alternative brain ageing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
snRNA-seq data and analysis output are available at the AD Knowledge Portal (https://adknowledgeportal.org). The AD Knowledge Portal is a platform for accessing data, analyses and tools generated by the Accelerating Medicines Partnership (AMP-AD) Target Discovery Program and other National Institute on Aging (NIA)-supported programs to enable open-science practices and accelerate translational learning. The data, analyses and tools are shared early in the research cycle without a publication embargo on secondary use. Data are available for general research use according to the following requirements for data access and data attribution (https://adknowledgeportal.org/DataAccess/Instructions). Access to the content described in this Article is available online: the Synapse database for raw and processed snRNA-seq data (https://www.synapse.org/#!Synapse:syn31512863); the online portal for data browsing (https://github.com/naomihabiblab/BEYOND_DLPFC); the Synapse database for bulk RNA-seq dataset (https://www.synapse.org/#!Synapse:syn3388564); and the Synapse database for the spatial transcriptomics (Visium) dataset (https://www.synapse.org/#!Synapse:syn62110225). Other ROSMAP resources can be requested at the RADC Resource Sharing Hub (https://www.radc.rush.edu). Source data are provided with this paper.
Code availability
The complete code base used in this study is available at GitHub (https://github.com/naomihabiblab/BEYOND_DLPFC) and includes library-level snRNA-seq analysis to remove background RNA, quality control steps, cell type classification and doublet annotation; cell-type-level snRNA-seq analysis to remove low-quality data, subclustering analysis and functional annotations; BEYOND algorithmic approach of visualizing the cellular landscape, fitting subpopulation and trait dynamics, and defining cellular communities; additional analyses presented in this study, such as CelMod fitting and trait associations, causal modelling and validations (smFISH, pTau and morphology analysis, as well as spatial transcriptomics subpopulation co-localization analysis); code for generating the Figures and Extended Data Figures; a guide to reproduce the graphs presented in the study from the Supplementary Tables.
References
Cain, A. et al. Multicellular communities are perturbed in the aging human brain and Alzheimer’s disease. Nat. Neurosci. 26, 1267–1280 (2023).
Mathys, H. et al. Single-cell atlas reveals correlates of high cognitive function, dementia, and resilience to Alzheimer’s disease pathology. Cell 186, 4365–4385 (2023).
Yang, A. C. et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 603, 885–892 (2022).
Pandey, S. et al. Disease-associated oligodendrocyte responses across neurodegenerative diseases. Cell Rep. 40, 111189 (2022).
Luquez, T. et al. Cell type-specific changes identified by single-cell transcriptomics in Alzheimer’s disease. Genome Med. 14, 136 (2022).
Sadick, J. S. et al. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer’s disease. Neuron 110, 1788–1805 (2022).
Tuddenham, J. F. et al. A cross-disease human microglial framework identifies disease-enriched subsets and tool compounds for microglial polarization. Preprint at bioRxiv https://doi.org/10.1101/2022.06.04.494709 (2022).
Gerrits, E. et al. Distinct amyloid-β and tau-associated microglia profiles in Alzheimer’s disease. Acta Neuropathol. 141, 681–696 (2021).
Habib, N. et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23, 701–706 (2020).
Grubman, A. et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22, 2087–2097 (2019).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017).
Mostafavi, S. et al. A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer’s disease. Nat. Neurosci. 21, 811–819 (2018).
Zhang, B. et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153, 707–720 (2013).
Mathys, H. et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337 (2019).
Lau, S.-F., Cao, H., Fu, A. K. Y. & Ip, N. Y. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 117, 25800–25809 (2020).
Zhou, Y. et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 26, 131–142 (2020).
Garcia, F. J. et al. Single-cell dissection of the human cerebrovasculature in health and disease. Nature 603, 893–899 (2022).
Dubois, B. et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 9, 1118–1127 (2010).
Bennett, D. A., Schneider, J. A., Arvanitakis, Z. & Wilson, R. S. Overview and findings from the religious orders study. Curr. Alzheimer Res. 9, 628–645 (2012).
Bennett, D. A. et al. Overview and findings from the Rush Memory and Aging Project. Curr. Alzheimer Res. 9, 646–663 (2012).
Habib, N. et al. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat. Methods 14, 955–958 (2017).
Habib, N. et al. Div-Seq: single nucleus RNA-seq reveals dynamics of rare adult newborn neurons. Science 353, 925–928 (2016).
Bennett, D. A. et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66, 1837–1844 (2006).
The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol. Aging 18, S1–S2 (1997).
Kang, H. M. et al. Multiplexed droplet single-cell RNA-sequencing using natural genetic variation. Nat. Biotechnol. 36, 89–94 (2018).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Waller, R., Mandeya, M., Viney, E., Simpson, J. E. & Wharton, S. B. Histological characterization of interneurons in Alzheimer’s disease reveals a loss of somatostatin interneurons in the temporal cortex. Neuropathology 40, 336–346 (2020).
Bateman, R. J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367, 795–804 (2012).
Jack, C. R. et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Selkoe, D. J. & Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608 (2016).
Carpenter, A. E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006).
Felsky, D. et al. Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat. Commun. 10, 409 (2019).
Setty, M. et al. Characterization of cell fate probabilities in single-cell data with Palantir. Nat. Biotechnol. 37, 451–460 (2019).
Stassen, S. V., Yip, G. G. K., Wong, K. K. Y., Ho, J. W. K. & Tsia, K. K. Generalized and scalable trajectory inference in single-cell omics data with VIA. Nat. Commun. 12, 5528 (2021).
Iturria-Medina, Y. et al. Unified epigenomic, transcriptomic, proteomic, and metabolomic taxonomy of Alzheimer’s disease progression and heterogeneity. Sci. Adv. 8, eabo6764 (2022).
Bennett, D. A. et al. Religious Orders Study and Rush Memory and Aging Project. J. Alzheimers Dis. 64, S161–S189 (2018).
Boyle, P. A. et al. Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann. Neurol. 85, 114–124 (2019).
Boyle, P. A. et al. To what degree is late life cognitive decline driven by age-related neuropathologies? Brain 144, 2166–2175 (2021).
Yu, L. et al. Neuropathologic correlates of human cortical proteins in Alzheimer disease and related dementias. Neurology 98, e1031–e1039 (2022).
Wilson, R. S. et al. Education and cognitive reserve in old age. Neurology 92, e1041–e1050 (2019).
Wilson, R. S., Wang, T., Yu, L., Bennett, D. A. & Boyle, P. A. Normative cognitive decline in old age. Ann. Neurol. 87, 816–829 (2020).
Grodstein, F., Wang, T., Leurgans, S. E., Wilson, R. S. & Bennett, D. A. Modifiable psychosocial risk factors and delayed onset of dementia in older populations: analysis of two prospective US cohorts. BMJ Open 12, e059317 (2022).
De Jager, P. L. et al. A genome-wide scan for common variants affecting the rate of age-related cognitive decline. Neurobiol. Aging 33, 1017.e1–1017.e15 (2012).
Schneider, J. A., Arvanitakis, Z., Bang, W. & Bennett, D. A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204 (2007).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Mirra, S. S. et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41, 479–479 (1991).
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Fleming, S. J. et al. Unsupervised removal of systematic background noise from droplet-based single-cell experiments using CellBender. Nat. Methods 20, 1323–1335 (2023).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Simon, N., Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J. Stat. Softw. 39, 1–13 (2011).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22 (2010).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337 (2019).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20, 163–172 (2019).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Gu, Z. & Hübschmann, D. SimplifyEnrichment: a Bioconductor package for clustering and visualizing functional enrichment results. Genom. Proteom. Bioinform. 21, 190–202 (2023).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Lee, A. J. et al. Multi-region brain transcriptomes uncover two subtypes of aging individuals with differences in Alzheimer risk and the impact of APOEe4. Preprint at bioRxiv https://doi.org/10.1101/2023.01.25.524961 (2023).
Lawlor, D. A., Harbord, R. M., Sterne, J. A. C., Timpson, N. & Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27, 1133–1163 (2008).
Tingley, D., Yamamoto, T., Hirose, K., Keele, L. & Imai, K. mediation: R package for causal mediation analysis. J. Stat. Soft. 59, 1–38 (2014).
Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 48, 1–36 (2012).
Virshup, I., Rybakov, S., Theis, F. J., Angerer, P. & Wolf, F. A. anndata: annotated data. Preprint at bioRxiv https://doi.org/10.1101/2021.12.16.473007 (2021).
McInnes, L. et al. UMAP: uniform manifold approximation and projection. JOSS 3, 861 (2018).
van der Maaten, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008).
Wood, S. N. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 467, 673-686 (2004).
Maynard, K. R. et al. Transcriptome-scale spatial gene expression in the human dorsolateral prefrontal cortex. Nat. Neurosci. 24, 425–436 (2021).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Zhao, E. et al. Spatial transcriptomics at subspot resolution with BayesSpace. Nat. Biotechnol. 39, 1375–1384 (2021).
van Dijk, D. et al. Recovering gene interactions from single-cell data using data diffusion. Cell 174, 716–729 (2018).
Acknowledgements
We thank the individuals who donated their brain to research through the Rush University Alzheimer’s Disease Center. The work was supported by NIH RF1 AG057473 (P.L.D.J. and D.A.B.), U01 AG061356 (P.L.D.J. and D.A.B.), U01 AG046152 (P.L.D.J. and D.A.B.), R01 AG070438 (P.L.D.J.), R01 AG015819 (D.A.B.), U01 AG072572 (P.L.D.J.), R01AG066831 (to V.M.); NIH K23 AG062750 (to H.-S.Y.); NIH NIA K23AG062750 (H.-S.Y.); NIH NIA R01AG080667 (H.-S.Y.); CS-02018-191971; the Israel Science Foundation (ISF) research grant no. 1709/19, the European Research Council grant 853409, the MOST-IL-China research grant no. 3-15687 and the Myers Foundation (N.H.); An Alzheimer’s Association Grant through the AD Strategic Fund (ADSF-21-816675) (M.T. and H.-U.K.). A.K.S. was supported by a Minerva Fellowship of the Minerva Stiftung Gesellschaft fuer die Forschung mbH. Diagrams in Figs. 1, 3 and 5 and Extended Data Fig. 6 were created using BioRender.
Author information
Authors and Affiliations
Contributions
P.L.D.J. and N.H. designed the study. C.M. prepared the single-nucleus libraries and performed sequencing. M.F. performed the sequence alignment and demultiplexing analysis. G.S.G. performed the computational and statistical analyses, with guidance of N.H., H.-S.Y., V.M., P.L.D.J. and with the help of A.C., A.K.S., C.C.W. and L.Z.; H.-S.Y. performed the causality modelling analysis. M.T. performed smFISH, immunohistochemistry experiments and image analysis. M.T., A.S. and H.-U.K. generated the ST data. N.C.-L., H.-U.K. and G.S.G. analysed the ST data. Y.W. analysed the bulk RNA-seq data. G.S.G., H.-S.Y., V.M., N.H. and P.L.D.J. wrote the manuscript, and all of the co-authors provided critical comments. D.A.B. is principal investigator of the parent ROS and MAP studies and obtained funding and performed study supervision. P.L.D.J., A.R. and D.A.B. obtained funding for the project.
Corresponding authors
Ethics declarations
Competing interests
A.R. is a founder and equity holder of Celsius Therapeutics, an equity holder in Immunitas Therapeutics and, until 31 August 2020, was an scientific advisory board member of Syros Pharmaceuticals, Neogene Therapeutics, Asimov and Thermo Fisher Scientific. From 1 August 2020, A.R. is an employee of Genentech, a member of the Roche Group. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 snRNA-seq libraries preprocessing and quality controls (QCs).
(a) Distributions across batches of: sex, cognitive diagnosis, Braak stage and CERAD score. (b) QC pipeline selecting 465 participants for cell atlas and 437 participants for downstream analysis including snRNA-seq libraries with a sufficient number of robustly assigned nuclei. (c) UMAP embeddings of two example snRNA-seq libraries prior to any QCs. Dot colour: predicted cell type (left) and prediction uncertainty (Shannon entropy, right). (d) Example snRNA-seq library manually curated for cells of low-quality libraries. Based on the manual curation of 10 such libraries, cell-type-specific low-quality thresholds over the number of UMIs (#UMI) and the number of unique genes (#Genes) were chosen. (e,f) Distributions of (e) # UMI and (f) #Genes threshold for low quality nuclei per cell type in the manually curated libraries, indicating selected thresholds. (g-i) Detection of doublet cells. UMAP embedding of example library annotated by (g) demultiplexing doublet annotation (demuxlet algorithm), or (h) DoubletFinder doublet-likelihood scores. (i) Distribution of the Matthews correlation coefficient (MCC) scores, reflecting prediction sensitivity, specificity and precision, for a range of thresholds over the DoubletFinder scores, per library (separate line, top), and the maximizing threshold chosen per library (dots, bottom). (j) UMAP embedding of the example library (in c) post-QCs, coloured by cell-type prediction. (k) Distribution of number of nuclei per participant. Dash line = minimum number of nuclei for a participant in the Discovery sample. (l) The average number of UMIs per cell type in each participant; Dots: individual participants (n = 465 participants per cell type). (m) Number of nuclei per cell type and participant: absolute (left) and proportions (right). Coloured by cell classes. Box: 1st and 3rd quartile, line: median, whiskers extend from box to the highest and lowest values within 1.5 times the distance between the quartiles.
Extended Data Fig. 2 Glial subpopulation diversity.
(a,d,g) Subpopulation proportions across participants for (a) microglia, (d) astrocytes and (g) oligodendroglia cells. Dots: 465 individual participants. Box: 1st and 3rd quartiles; line: median, whiskers extend to the highest and lowest values within 1.5 times the distance between the quartiles. (b,e,h) QC measures. Distributions of number of UMIs and number of Genes detected for each subpopulation of (b) microglia, (e) astrocytes and (h) oligodendroglia. (c,f,i) Selected markers and top differentially expressed genes between subpopulations. Gene expression (columns) across subpopulations (rows) of (c) microglia, (f) astrocytes and (i) oligodendroglia. Dot colour: mean expression in expressing cells. Dot size: percent of cells expressing the gene.
Extended Data Fig. 3 Comparison of glial subpopulation to previously defined gene signatures.
Heatmap of previous signature genes (rows) for cell subpopulations (columns) of (a) microglia, (b) astrocytes and (c) oligodendroglia, separated by signature and split by reference source. Colour-scale: row scaled expression out of expressing cells. Genes defined in multiple signatures appear multiple times. (d) Comparison of previous gene signatures to subpopulations of the vascular niche. Scaled mean signature score of published gene signatures (columns) within each subpopulation (rows). (*) = Significantly enriched signatures (U-test, FDR<0.01). Published signatures from1,3,17.
Extended Data Fig. 4 Vascular niche- and neuronal subpopulation diversity.
(a,d,g) Subpopulation proportions across participants for (a) vascular niche cells, (d) inhibitory neurons and (g) excitatory neurons. Dots: 465 individual participants, box: 1st and 3rd quartiles, line: median, whiskers extend to the highest and lowest values within 1.5 times the distance between the quartiles. (b,e,h) QC measures. Distributions of number of UMIs and number of Genes detected for each subpopulation of (b) vascular niche, (e) inhibitory neurons and (h) excitatory neurons. (c,f,i) Selected markers and top differentially expressed genes between subpopulations. Gene expression (columns) across subpopulations (rows) of (c) vascular niche, (f) inhibitory neurons and (i) excitatory neurons. Dot colour: mean expression in expressing cells. Dot size: percent of cells expressing the gene.
Extended Data Fig. 5 Clustering quality and neuronal subtypes annotations.
(a) Quality measures of clustering showing the cohesion within each cluster and separation between clusters. For each cluster (row) showing the distribution of shared nearest neighbours assigned to other clusters (columns) For each cell class. Coloured by the fraction of shared nearest neighbours. Rows and columns are sorted by the cluster number. (b-c) Classification of (b) excitatory and (c) inhibitory neuronal cells, by previous annotations provided by the Allen Brain Map. Heatmaps of the percentage of cells from each of our clusters assigned to each of the provided annotations (Methods) linking neuronal sub-types to marker genes of inhibitory and excitatory neurons and to cortical layers (colour bar).
Extended Data Fig. 6 CelMod predictions and endophenotype associations.
(a) Evaluation of CelMod prediction of subpopulation proportions in bulk RNA samples with matching snRNA-seq measurements in the same participant (n = 419 samples). Spearman correlation between snRNA-seq (actual) and CelMod bulk-predicted proportions, over the held-out set (test set) of participants (Methods). * = FDR corrected p-value. (b) Comparison of the estimated effect sizes regressing endophenotypes on subpopulation proportions, for the snRNA-seq (Discovery cohort, x-axis) and the bulk predictions (Replication cohort, y-axis). n = 419. The Spearman correlation between the effect sizes and FDR corrected p-value are shown for each comparison. (c) Associating subpopulation proportions to endophenotypes: CERAD score, Braak stage and AD dementia (linear regression controlled for cofounders, FDR<0.05, Methods), showing subpopulations significantly associated with at least one of the tested traits in one on the cohorts: Discovery (left, n = 437), Replication (centre, n = 673) and the meta-analysis of both cohorts (right, n = 1,110). Colour scale: t-stat. (d-h) Causal mediation models which together with Fig. 3f–i position Mic.12, Mic.13, Ast.10 and Oli.7 within the Aβ→tau→cognitive decline AD cascade, indicating direct and mediated effects, as well as proportion of effect mediated. Number of participants: (d,e,g) n = 432, (f) n = 413, (h) n = 433. (i) Validation of the structural equation model (SEM; as in Fig. 3j) in the CelMod predicted subpopulation proportions of the Replication cohort. Arrows show association directionality and relative strength.
Extended Data Fig. 7 smFISH quantification analysis.
(a) Gene expression (columns) across microglial subpopulations (rows) for selected differential genes and known markers. Dot colour: column-scaled mean expression of expressing cells. Dot size: percentage of expressing cells. (b) Gene expression of markers used for smFISH across microglial subpopulations. Dot colour and size as in (a). (c) A gallery of representative RNAscope smFISH data showing split channels by marker: CPMhigh Mic.12, TPRG1high Mic.13, and a MRC1high macrophage, together with IBA1 (green) and DAPI (blue) staining. (d) Bivariate expression distributions in snRNA-seq (top) and smFISH (bottom), coloured by assigned snRNA-seq subpopulations (top) predicted assignment in smFISH data (bottom, Methods).
Extended Data Fig. 8 Robustness of cellular landscape modelling by BEYOND.
(a) 3D PHATE embedding of all 437 snRNA-seq participants, coloured by clustering of participants based on their cellular environments (Methods). (b) Distinct patterns of subpopulations along the cellular landscape manifold, showing additional subpopulations to those of Fig. 5c. Participants (dots) are coloured by the locally smoothed proportion of each subpopulation. (c) Robustness of the cellular landscape to the embedding method and set of subpopulations used in BEYOND. Participants are coloured by the locally smoothed subpopulation proportion. (d-e) Visualizing fitted pseudotime, trajectories and Shannon entropy of trajectory probabilities outputted by: (d) VIA (n = 437 participants), and (e) Palantir algorithms (n = 386, excluding participant-clusters #9 and #10 in a).(f) Robustness of trajectories and pseudotime predictions using different algorithms (over the overlapping n = 386 participants). (Top) Pseudotime assigned for each individual by Palantir compared to VIA. (Bottom) Trajectory probabilities Pearson correlations. Corrected for multiple hypothesis testing (BH). (g) Participants’ trajectory probabilities entropy drop along pseudotime, in the Palantir model. Dots are coloured by prAD minus ABA trajectory probabilities. The grey area indicates a pseudotime range (0, 0.11) in which the two trajectories are not well separated. (h) Trait-dynamics of AD-related traits along the pseudotime in each of the inferred trajectories, showing the datapoints used in fitted curves and error bands showing 0.95 CI (Methods). n = 386 participants. As in Fig. 5f. (i-j) Validation of cellular landscapes and trajectories using the Replication cohort (n = 673 non-overlapping participants, with the 62 reliable CelMod bulk-predicted subpopulations proportions to represent cellular environments). (i) The Palantir model over replication landscape as in (e). (j) Trajectory probabilities entropy drop as in (g) but over the replication landscape.
Extended Data Fig. 9 Cellular dynamics and communities.
(a) Distinct patterns of subpopulation dynamics, as Fig. 6a with datapoints used to fit the curves. Dots are participants (n = 386). Error bands: 0.95 CI. (b) Graph of multi-cellular communities. Nodes: Subpopulations. Edges: co-occurrences (Spearman correlation, green-purple scale) or dynamics similarity (white-red scale). Excluding edges of low similarity (correlation (−0.2, +0.4), dynamics (−0.2, +0.2)) for visualization. See Fig. 6b. (c) Distinct dynamic patterns for cellular communities across trajectories. Whole-community dynamics of community proportion along pseudotime in each trajectories. Dots are individual participants (n = 386). See Fig. 6c. Error bands: 0.95 CI. (d) Validation in the Replication cohort of coordinated subpopulation dynamics along trajectories. Presented as in Fig. 6a, but over the replication landscape. Error bands: 0.95 CI. (e-g) Spatial transcriptomics (ST, Visum, Methods) validations of cellular communities. Dots are individual participants (n = 637). (e) Discovery cohort participants included in ST validations (n = 10), and their assignment to trajectories (annotated as: Early, prAD or ABA). 2D PHATE embedding as in Fig. 5b. (f) Pearson correlation between Mic.13 and Ast.10 gene signature expression across the Visium spots for each of the participants, grouped by their trajectory assignment. Dots: signature expression in visium spots. Line: regression line. Error bands: 0.95 CI of regression line. P-value: one-sided T-test (positive association), FDR corrected for multiple hypothesis, dot fill colour indicates significance of correlation. (g) Divergent association of Ast.10 and Ast.5 between the two trajectories. Distribution of Ast.10 and Ast.5 gene signature expression for each participant (n = 10), grouped by their trajectory assignment. Per participant, significance of the differences of means were tested by one-sided t-test: (1) for prAD participants: Ast.10 levels being strictly higher than Ast.5 levels, and (2) for ABA participants: Ast.5 levels being significantly higher than Ast.10 levels (Methods). Significance level is shown by the dot fill colour.
Supplementary information
Supplementary Table 1
Clinicopathological characteristics of participants in discovery (snRNA-seq, 465 participants) and replication (non-overlapping bulk RNA-seq, 673 participants) cohorts.
Supplementary Table 2
Atlas characterization: differential expression and pathway analysis of subpopulation versus all other subpopulations, and pairwise analysis of subpopulation versus subpopulation for microglia and astrocytes.
Supplementary Table 3
Endophenotype associations: subpopulation proportions, CelMod correlations and predicted proportions, snRNA-seq, CelMod predicted and meta-analysis for subpopulation–endophenotype associations.
Supplementary Table 4
smFISH results: smFISH marker expression, subpopulation predicted probabilities, endophenotype and morphological quantification, as well as subpopulation proportions per participant.
Supplementary Table 5
BEYOND results: cellular landscape embedding, trajectories, endophenotype and subpopulation dynamics, subpopulation community assignment, community proportions per participant, community dynamics and endophenotype associations.
Supplementary Table 6
Spatial transcriptomics validations: Mic.13–Ast.10 and Ast.10–Ast.5 colocalization results.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Green, G.S., Fujita, M., Yang, HS. et al. Cellular communities reveal trajectories of brain ageing and Alzheimer’s disease. Nature 633, 634–645 (2024). https://doi.org/10.1038/s41586-024-07871-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07871-6
This article is cited by
-
Divergent disruptive effects of soluble recombinant tau assemblies on synaptic plasticity in vivo
Molecular Brain (2025)
-
Comparative assessment of the Sikun 2000 sequencing platform for whole genome sequencing
Scientific Reports (2025)
-
Synaptic vulnerability to amyloid-β and tau pathologies differentially disrupts emotional and memory neural circuits
Molecular Psychiatry (2025)
-
Microglia heterogeneity, modeling and cell-state annotation in development and neurodegeneration
Nature Neuroscience (2025)
-
PGRN as an emerging regulator of lipid metabolism in neurodegenerative diseases
Communications Biology (2025)