Abstract
The RNA-binding protein CsrA regulates the expression of hundreds of genes in several bacterial species, thus controlling virulence and other processes. However, the outcome of the CsrA-mRNA interactions is modulated by competing small RNAs and other factors through mechanisms that are only partially understood. Here, we show that CsrA accumulates in a dynamic membraneless compartment in cells of E. coli and other pathogenic species. In addition to CsrA, the compartment contains components of the RNA-degrading complex (degradosome), regulatory small RNAs, and selected mRNAs. Formation of the compartment is associated with a switch between promoting and repressing virulence gene expression by CsrA. We suggest that similar CsrA switches may be widespread in diverse bacteria.
Similar content being viewed by others
Introduction
The human-specific pathogens enteropathogenic and enterohemorrhagic Escherichia coli (EPEC and EHEC), also known as “attaching and effacing” (AE) pathogens, comprise a subset of E. coli strains responsible for foodborne infections with potentially life-threatening complications1. The primary virulence mechanism of AE pathogens is a type III secretion system (T3SS), which consists of a syringe-like complex made up of components encoded by 41 genes clustered in the Locus of Enterocyte Effacement (LEE) and organized into several operons termed LEE1 to LEE72,3. The T3SS acts as a delivery device, enabling host-attached bacteria to inject dozens of effector proteins into the host cytoplasm. These effector proteins manipulate multiple host processes to the pathogen’s advantage4. The expression of the T3SS genes in AE pathogens is subject to complex transcriptional and post-transcriptional regulation5,6,7,8. Among the latter, the RNA-binding protein Carbon storage regulator A (CsrA) has emerged as a vital post-transcriptional virulence regulator9.
CsrA is a small (6.8 kDa), conserved protein that forms compact homodimers containing two identical RNA-binding sites10. It functions by binding to mRNAs containing two or more motifs, consisting of a GGA single-stranded loop sequence in a context of a stem-loop structure11. The outcome of CsrA-mRNA interaction is context-dependent12. For example, it can inhibit translation when its binding site overlaps with the ribosome binding site13, but it can also promote translation by stabilizing the bound mRNA14. The small RNAs (sRNAs) CsrB and CsrC, each containing multiple CsrA binding sites, modulate CsrA activity by competition with mRNA targets15,16. Their decay rate is controlled, among others, by the RNA degrading machinery, the degradosome complex17,18. The Csr proteins and sRNAs, collectively known as the Carbon Storage Regulator (CSR) system, regulate complex processes such as metabolism, biofilm formation, production of secondary messengers, motility, and virulence in many bacteria12,19,20,21. In AE pathogens, CsrA fulfills a particularly complex role, functioning as both a positive and negative regulator of T3SS biogenesis9. Furthermore, CsrA is essential for sensing bacterial attachment to mammalian host cells and bacterial adaptation to a host-attached lifestyle22.
AE pathogens must activate their T3SS expression soon after oral uptake, with a major host cue in making this decision being a temperature shift to 37 °C23. This ensures the rapid completion of T3SS biogenesis, a task spanning two generations3. Only then the bacteria can attach and successfully interact with the intestinal epithelial cells to establish colonization. An equally important decision is when to shut down the costly T3SS expression24. For example, if the pathogen enters the stationary phase before achieving direct contact with host cells, it is crucial to repress the expensive T3SS production to save resources and improve fitness. Here, we tackle the molecular basis for this decision-making process using EPEC as the model system. We show that this decision is orchestrated by the assembly of a membraneless compartment via CsrA-RNA interactions. Analysis of the highly enriched compartment shows that it is composed of components of the Csr and degradosome systems and acts by recruiting specific sets of sRNAs and mRNAs, which are spatially regulated within this compartment. Our data, along with the conservation of the Csr system, suggest that similar mechanisms are involved in controlling virulence and other complex behaviors across the bacterial kingdom.
Results
Formation of CsrA foci by Gram-negative pathogens
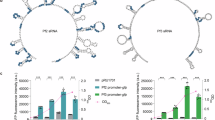
We previously showed that CsrA plays a key role in the adaptation of EPEC to a cell-adherent lifestyle22. Yet, the role of CsrA in pathogens that fail to contact the host cell remains unexplored. To address this knowledge gap, we investigated CsrA expression dynamics under conditions mimicking growth in the host intestine, in the absence of host cells. To this aim, we introduced a gfp gene into the EPEC genome, creating csrA-gfp transcriptional and translational fusions (Fig. 1A). We then confirmed that the CsrA-GFP hybrid protein is expressed and functional (Fig. S1A–C). Surprisingly, when testing for CsrA-GFP expression by microscopy we observed the emergence of distinct CsrA-GFP foci, which were absent from the strain carrying csrA-gfp transcriptional fusion (Fig. 1B, C). Similar foci were observed when CsrA was fused to other fluorescent proteins (Fig. S2A), and when FLAG-tagged CsrA was examined using immunofluorescent microscopy (Fig. S2B), indicating that foci formation is an intrinsic property of CsrA. Typically, a single dominant CsrA focus per bacterium was observed, displaying variable sizes and generally localized to the cell periphery (Figs. 1B, D and S2). During cell division, this focus was inherited by one daughter cell (Fig. S3), possibly accounting for the lack of foci in some bacteria (Fig. 1B, C).
A Schematics of the full-length csrA gene, translationally (a) or transcriptionally (b) fused to gfp. Boxes represent translated genes, and the solid green line represents an untranslated region containing a synthetic ribosomal binding site (RBS). Both fusions are located at the native chromosomal locus of the csrA gene. Black arrows and lines represent the native regulatory region (PcsrA) and 5’UTR of csrA, respectively. B Formation of CsrA-GFP foci in EPEC. EPEC strains containing csrA-gfp and csrA-rbs-gfp (strains NE9077 and NE9121 shown in A) were grown in DMEM to OD600 0.5. Next, bacterial cells were fixed, washed, and visualized by fluorescence microscopy. Scale bar 2 µm. The two right panels show enlarged sections marked in the merge panels. C Quantification of CsrA foci. Bacteria were grown and fixed as in (B). The percentage of foci containing bacteria was determined by microscopy. For each experiment, n ≥ 200 bacteria were analyzed in each sample. Data presented as mean values and standard deviation of three independent experiments, with an unpaired two-tailed t-test p-value = 0.0023 (**) between the compared samples. ND not-detectable. D Distribution of focus sizes. Bacteria were grown and processed as described in (B). Analysis of CsrA-GFP foci area size was done using the NIS Elements software. In each biological repeat, n > 400 bacterial focus areas were measured. The largest focus area detected in the same biological repeat was set as 100%. The relative percentage range of the focus size is indicated in the X-axis. Data presented as mean values and standard deviation of three biological repeats. E Formation of CsrA foci by enteropathogens. Cultures of EPEC, C. rodentium (C.R.), Y. pseudotuberculosis (Y.P.), and V. cholerae (V.C.), expressing CsrA-GFP translational fusion from an inducible plasmid were grown to OD 0.45–0.6. Induction was done in the final hour of growth. In the case of S. typhimurium (S.T), csrA-gfp is translationally fused on the chromosome (strain LA11509). All samples were processed and visualized as in (B) and quantified as in (C). The Y axis represents the average percentage of focus containing bacteria and the error bars represent the standard deviation of 3 different images taken from each sample. n ≥ 200 bacteria. ND non-detectible. F Representative images of the experiment described in (E). Scale bar 2 µm.
To test whether the formation of CsrA foci is unique to EPEC or represents a wider phenomenon, we probed for CsrA localization in additional pathogens that rely on CsrA to regulate their virulence. These include Salmonella (i.e., Salmonella enterica serovar Typhimurium), Citrobacter rodentium, Yersinia pseudotuberculosis and Vibrio Cholerae21,25. In all cases, but that of Y. pseudotuberculosis, a similar formation of CsrA foci was detected (Fig. 1E, F), indicating that the formation of CsrA foci is conserved and widespread. We decided to focus on the EPEC model to decipher the significance of this phenomenon and its association with virulence regulation.
CsrA equilibrium between the focus and cytoplasm compartments
CsrA foci were visible when the EPEC culture reached a density of OD600 0.2, with foci sizes significantly increasing as the density progressed (Fig. 2A, B). To elucidate the dynamics of the CsrA equilibrium between the foci and cytoplasmic compartments (CsrA focus/cytoplasm ratio), we monitored the relative fluorescent intensities/area, which is proportional to the concentration of CsrA-GFP in these two compartments throughout growth. A significant shift in the CsrA focus/cytoplasm ratio during growth, coupled with the augmentation in foci size and intensity was unveiled (Fig. 2B, C). In contrast, the intensity/area of cytoplasmic CsrA-GFP remained constant (Fig. 2D). These findings indicate that during growth, CsrA localizes to two distinct compartments: focus, characterized by escalating size and CsrA local concentration, and the cytoplasm, where CsrA concentration remains lower and constant. Given the central role of CsrA in virulence regulation, we then asked what might be the role of the CsrA focus in controlling virulence, what is the composition of the focus, and how it is generated.
A Formation and enlargement of CsrA foci over time. EPEC csrA-gfp (strain NE9077) was grown in DMEM to the indicated densities (OD600), fixed, washed, and visualized by fluorescence microscopy. Scale bar 2 µm. B Distribution of foci intensity over time. Bacteria were grown as in (A) and images were analyzed using Image J software. Bacteria lacking CsrA foci were eliminated from this analysis. In the subpopulation of foci-containing bacteria, the sum of focus intensities was calculated. For each sample, n > 400 bacterial foci were analyzed. The X-axis represents the relative intensity (%) compared to the focus containing the highest intensity in a single experiment (set as 100%). The Y-axis represents the percentage of foci counted for each intensity range. Data presented as mean values and standard deviation of three biological repeats, with multiple t-tests (unpaired two-tailed) done between OD 0.2 and OD 1 samples, indicating significant differences with a t-test p-value < 0.01 (**) and t-test p-value < 0.001 (***). C The relative CsrA-GFP intensity in the foci and cytoplasm. EPEC expressing CsrA-GFP (strain NE9077) was grown to the indicated densities (OD600) and the sum intensity per constant area (correlates with relative concentration) of cytoplasmic and CsrA-GFP focus was determined for each bacterium (n ≥ 100 bacteria for each measurement), using Image J. The X-axis shows the average foci/cytoplasm ratio of relative CsrA-GFP intensities. The Y-axis indicates the percentage of bacteria belonging to the different categories. Data presented as mean values and standard deviation of three independent biological repeats, with multiple t-tests done between OD 0.2 and OD 1 samples, indicating significant differences with an unpaired two-tailed t-test p-value = 0.0002 (***), 0.0001 (***), and 0.0005 (***) for foci/cytoplasm intensity ratios of: 1-5, 6-10, and >10, respectively. D The cytoplasmic CsrA-GFP relative intensities. EPEC was grown to the indicated densities and the relative GFP intensity in the cytoplasm was determined as in (C). The results were normalized to the cytoplasmic GFP signal obtained at OD 0.2. Data presented as mean values and standard deviation of three independent biological repeats, n ≥ 100 bacteria were measured from each sample, ns- not significant (Unpaired two-tailed t-test between OD 0.2 and OD 0.6 p-value = 0.189 and between OD 0.2 and OD 1 p-value = 0.205).
CsrA-RNA interactions drive focus formation
CsrA functions as a homodimer, containing two identical domains that bind specific sites on target RNAs10,26. We assumed that the interaction of the divalent CsrA with RNA sets containing multiple CsrA binding sites could result in focus formation. To test this premise, we tracked the foci formation capacity by CsrA mutants deficient in RNA binding. Since CsrA is important for EPEC fitness, we circumvented the complication of using a csrA null EPEC mutant by generating merodiploid strains co-expressing two csrA alleles, both under their native promoter (Fig. 3A). The first allele encodes for GFP fused to wild type CsrA, or mutated CsrA, deficient in RNA binding (CsrA-GFP and CsrAR44A-GFP, respectively)11,27. The second allele encodes for wild-type CsrA tagged with a FLAG epitope (CsrA-FLAG). CsrA-GFP and CsrA-FLAG were biologically functional (Fig. S1A, S1D), and could form dimers, as affirmed by co-immunoprecipitation experiments, revealing that both CsrA-GFP and CsrAR44A-GFP co-precipitated with CsrA-FLAG (Fig. 3B). These results indicate the co-existence of three CsrA complexes in each merodiploid EPEC bacterium: i) CsrA-FLAG homodimers, ii) CsrA-GFP or CsrAR44A-GFP homodimers, and iii) heterodimers of CsrA-FLAG with either CsrA-GFP or CsrAR44A-GFP. Importantly, the subsequent microscopy-based examination showed that EPEC expressing CsrA-GFP formed typical foci, whereas bacteria expressing CsrAR44A-GFP did not (Fig. 3C), indicating that the formation of foci depends on CsrA dimers containing two functional RNA-binding domains. To reinforce this conclusion, merodiploid strains co-expressing untagged native CsrA and GFP fused to either native CsrA or CsrA mutants deficient in RNA binding (CsrAR44A or CsrAL4A) were generated11,27. In line with our results, EPEC expressing CsrAR44A or CsrAL4A were strongly attenuated in foci formation while maintaining similar expression levels of CsrA-GFP (Fig. 3D–F). Similar trends of CsrA and CsrAR44A were recorded also using E. coli strain MG1655 (Fig S4). While CsrAR44A-GFP expressed from its native chromosomal promoter could not be detected in the foci (Fig. 3C–E), its overexpression from a plasmid led to its partial recruitment to the foci, likely as a heterodimer with wild-type CsrA, thereby harboring a single RNA-binding site (Fig. S5). In this case, the heterodimer increased concentration appears to compensate for its mono-valency enabling recruitment to the foci. These results signify that RNA interaction with divalent CsrA dimers is the major driving force for foci assembly.
A Schematics of merodiploid csrA genes carried by EPEC strains. These include csrA wild type (a), or csrA mutant (csrAR44A) deficient in RNA binding (b), fused to gfp (csrA-gfp and csrAR44A-gfp, respectively). The second allele is FLAG tagged csrA (csrA-flag). All of the alleles are localized to the native chromosomal site and expressed under the native csrA regulatory region. Boxes represent translated genes, and black lines and arrows represent the native promoters and 5’UTR of csrA, respectively. B CsrA-GFP and CsrAR44A-GFP and CsrA-FLAG form heterodimers. EPEC strains with the indicated genotypes (strains NE9361, NE9362, NE9142, NE9133) were grown in DMEM to OD 0.5. Next, Bacteria were lysed and subjected to immunoprecipitation with anti-FLAG antibodies, followed by Western blotting using anti-GFP and anti-FLAG antibodies. Stain-free gel of total protein was used as loading control (Control). Cleared lysate (Input), unbound (UB) and immunoprecipitated (IP) fractions were analyzed. C The two strains indicated in (A) were grown as in (B), fixed, and visualized by fluorescence microscopy, Scale bar 2.5 µm. The two right panels show the enlarged sections marked in the middle panels. D Merodiploid EPEC strains expressing wild-type CsrA as well as GFP fused to either wild-type CsrA, or CsrA mutants (L4A and R44A), deficient in RNA binding (NE9133, NE9141, and NE9142), were grown and processed as in (A). Scale bar 1 µm. E Quantification of the CsrA-GFP foci produced by the three merodiploid EPEC strains as described in (D). Data presented as mean values and standard deviation of three independent biological repeats, with an unpaired two-tailed t-test p-value = 0.0011 (**) between the WT vs L4A and p-value = 0.002 (**) between WT and R44A. ND not detectable. F The amount of CsrA-GFP, CsrAR44A-GFP, and CsraL4A-GFP in the three merodiploid EPEC strains shown in (D) was determined by Western blot using an anti-GFP antibody. Stain-free gel of total protein was used as loading control (Control).
Interactions of CsrA with multivalent sRNAs facilitate focus formation
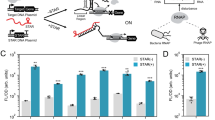
We next sought to identify the RNAs that interact with CsrA to generate foci. The CsrA regulators CsrB and CsrC are sRNAs containing 22 and 12 CsrA binding sites, respectively12,15,16, and thus are prime candidates for promoting focus formation through cross-bridging the divalent CsrA. Therefore, we asked whether CsrB and CsrC are localized to the CsrA foci. The localization of these RNAs was tested by microscopy using EPEC expressing the RNA-binding protein MS2 fused to GFP (MS2-GFP), and CsrB or CsrC tagged with an MS2 Binding Sequence (CsrBMS2-tag and CsrCMS2-tag) (Fig. 4A). We found that the expressed MS2-GFP and CsrA foci were colocalized exclusively in bacteria expressing CsrBMS2-tag or CsrCMS2-tag RNAs (Fig. 4A). As controls, expression of MS2-GFP alone, or its co-expression with untagged CsrB sRNA, or with MS2-tag RNA, did not result in colocalization with the CsrA focus (Fig. S6). This data indicates that both CsrB and CsrC are localized to CsrA foci.
A CsrB and CsrC are localized to the CsrA foci. EPEC expressing native CsrA fused to mScarlet (CsrA-mScarlet, strain LA10114) was transformed with two compatible plasmids: i) a plasmid encoding CsrB, or CsrC, sRNAs tagged with MS2 binding sites (CsrBMS2-tag, CsrCMS2-tag, plasmids pLA10176 and pLA10177), and ii) a plasmid expressing MS2-GFP under tet promoter (pLA11940), and constitutively expressed lacI gene. Bacteria were grown in infection-mimicking conditions and treated with IPTG to induce CsrBMS2-tag and CsrCMS2-tag. The MS2-GFP protein expression via a TetR-regulated promoter was induced in the last hour of growth. Bacterial cells were then fixed and visualized by fluorescence microscopy. Scale bar 2 µm. B CsrB is essential for the formation of CsrA foci in the early logarithmic growth phase. EPEC expressing CsrA-GFP (strain NE9077), or isogenic mutants deleted of csrB, or csrC, or both (NE9134, NE9135 and NE9137 respectively) were grown in DMEM to the indicated densities. Then, bacteria were fixed and visualized by fluorescence microscopy. Scale bar 1 µm. C Quantification of the experiment described in (B). Data presented as mean values and standard deviation from two independent experiments, t-test (unpaired two-tailed) was used to compare samples of OD 0.2, with a p-value = 0.0019 (**) between wt and ΔcsrB strains, p-value = 0.231 (ns) between wt and ΔcsrC, and p-value = 0.03 (*) between wt and ΔcsrB, ΔcsrC strains. n ≥ 200 bacteria were measured from each sample in all independent experiments. D CsrA levels in the strains used in (B). Proteins were extracted from the cultures shown in (B) and the levels of CsrA-GFP were evaluated by western blot using anti-GFP antibody. The culture densities and genotypes are indicated above the lanes. Total protein staining was used as loading control (LC) and a segment of the gel is shown. E CsrA-GFP levels in wild-type EPEC grown to different growth phases in DMEM. Bacteria were grown to different ODs and levels of CsrA-GFP were assessed by western blot (as in D). Total protein staining was used as loading control (Control) and a segment of the gel is shown. The relative average amount of CsrA-GFP in three biological repeats is shown below a representative blot. F CsrB levels in wild-type EPEC grown to different growth phases in DMEM. Bacteria were grown to different ODs, the total RNA was extracted and the relative amounts of CsrB was measured using RT-PCR. Data presented as mean values and standard deviation of four biological repeats (bars) and P-value (unpaired two-tailed t-test) is indicated.
To test whether CsrB and CsrC are required for focus formation or merely recruited to the CsrA foci, we compared the formation of foci by wild-type bacteria with that of mutants deleted of either csrB, csrC, or both. Foci formation was largely diminished in the ΔcsrB mutant, being very rare until reaching the late exponential growth phase (Fig. 4B, C). Upon reaching the late growth phase, CsrB-independent foci emerged but appeared fewer, fainter, and smaller than those generated in wild-type bacteria. Unlike CsrB, CsrC seemed dispensable for foci formation, and the ΔcsrB, ΔcsrC double mutant resembled the ΔcsrB single mutant (Fig. 4B, C). Complementing the ΔcsrB mutant with CsrB expressing plasmid, restored focus formation (Fig. S7), but ectopically expressed CsrC failed to do so (Fig. S8). In all cases, CsrA-GFP levels remained similar (Fig. 4D), implying that the lack of focus formation in the ΔcsrB mutant is unrelated to CsrA levels. To further probe for the contribution of CsrA and CsrB for foci formation we examined their relative levels during growth and found a small increase in CsrA, and a more pronounced increase in CsrB levels (Fig. 4E, F). These findings indicate that whereas both CsrB and CsrC localize to the focus, only CsrB exhibits a predominant role in focus formation, possibly due to having a higher number of CsrA binding sites. Interestingly, Y. pseudotuberculosis, which failed to produce CsrA foci (Fig. 1E), was reported to downregulate CsrB expression under the used growth conditions28.
The CsrA condensates contain degradosome components
To better understand the foci function, we aimed at elucidating their protein and RNA composition. To this end, we established a protocol for purifying intact CsrA foci (Fig. S9), and subjected them to proteome analysis using LC-MS/MS. Using two distinct methods, we unveiled Ribonuclease E (RNase E) and additional components of the degradosome complex, including polyribonucleotide nucleotidyltransferase (PNPase), enolase, and the DEAD-box RNA helicases RhlB and DeaD, as the most prominent proteins associated with the CsrA foci (Table 1, Supplementary Data 1, 2)29,30. These findings suggest that the foci contain the degradosome complex. To validate this notion, we sought to co-visualize CsrA and RNase E within the same bacterium. We engineered an EPEC strain expressing the native CsrA and RNase E fused to mScarlet and mNeon, respectively. As previously reported31, we observed that RNase E localized to the bacterial periphery, forming multiple small puncta outlining the bacterial circumference (Fig. 5A, Fig. S10). Importantly, the CsrA focus was significantly colocalized with one of the RNase E puncta (Fig. 5 and Fig. S11). We next tested whether the RNase E C-terminus, responsible for interaction with PNP, RhlB, and enolase32, is required for the formation of CsrA foci. We thus repeated the experiment using EPEC expressing CsrA-mScarlet and RNase E deleted of its C-terminus (rne-701 mutation32) and fused to mNeon (RNase E701-mNeon), both expressed from the native promoters. The levels of the truncated RNase E-mNeon were elevated in comparison to wild-type RNase E-mNeon, forming occasional large puncta (Fig S12A). The formation of CsrA foci in this strain persists, indicating that CsrA foci formation is independent of the RNase E C-terminus. Furthermore, CsrA foci remained significantly colocalized with the truncated RNase E puncta (Fig. S12). Of note, while RNase E controls the decay rate of CsrB18, we could not detect the reduced stability of the CsrA foci upon rifampicin treatment (Fig. S13). Conjointly, these findings corroborate the proteome results, reinforcing the premise that CsrA focus might be a regulatory hub, containing components of the CSR system and the degradosome complex, both of which are major posttranscriptional regulatory systems, widespread in the bacterial kingdom.
A Co-visualization of RNase E puncta and CsrA foci. EPEC strain expressing native CsrA fused to mScarlet (CsrA-mScarlet) and native RNase E fused to mNeon (RNase E-mNeon) (strain LA10288) were grown in DMEM to OD 0.3. Next, bacterial cells were fixed and visualized by fluorescence microscopy. Scale bar 1.0 µm. Arrows indicate colocalization (blue) or lack of colocalization (orange) of RNase E puncta and CsrA foci. B Visualization of CsrA-mScarlet focus and RNase E-mNeon punctum in a single bacterium. The experiment was done as in A, Scale bar 1.0 µm. The blue line indicates the location of the CsrA focus overlapping one of the RNase E-mNeon puncta. The pink line indicates the location of an RNase E-mNeon punctum that does not colocalize with the CsrA focus. C Colocalization pattern of the RNase E punctum and CsrA focus (marked with blue and pink lines in (B)). Fluorescence intensity patterns across the lines were determined using Image J. Correlations across the blue line (left panel) and pink line (right panel) are shown. For each panel, the left Y axis refers to CsrA-mScarlet and the right Y axis to RNase E-mNeon. D Quantification of CsrA-RNase E colocalization. Using the methodology shown in (B) and (C) 30 bacteria were analyzed in three biological repeats (10 bacteria in each repeat). The data was plotted and the Y-axis for CsrA shows the Pearson correlation coefficient between CsrA foci and RNase E puncta, while the Y-axis for RNase E shows the correlation between RNase E puncta and CsrA. Data are presented as box and whisker plot (median, box: first and third quartiles, and whisker: minimum and maximum). Statistics were done using an unpaired two-tailed t-test, p-value < 0.0001 (****). E Colocalization of natively distributed CsrA foci with native RNase E puncta (Sample), versus colocalization of simulated random CsrA foci distribution and native RNase E (Random) was determined as described in Fig. S11. The graph compiles the data of four biological repeats. In each repeat, 400–2000 bacteria were analyzed. Data presented as mean values and standard deviation of the four biological repeats, with an unpaired two-tailed t-test p-value = 0.02 (*).
The CsrA focus contains selected sRNAs and mRNAs
To discover the foci-associated RNAs, we extracted RNA from enriched CsrA foci and performed RNA-seq analysis. We found that the foci are significantly enriched with CsrB and CsrC (Fig. 6A and Supplementary Data 3), consistent with our microscopy-based analysis (Fig. 4A). CsrB accounted for 35-36% of the reads of the foci-associated RNAs (Supplementary Data 3), emphasizing it as a primary component of the focus. Seven additional non-coding regulatory sRNAs were significantly enriched in the CsrA foci (Fig. 6A, B and Supplementary Data 3), all but AspX contained at least two confirmed33,34 or putative CsrA binding sites (i.e., GGA triplet sequence). Selected mRNAs that were significantly enriched in the foci encode for genes involved in metabolism, iron uptake, and virulence (Fig. 6B, Supplementary Data 3). Comparing our results with the previously published CsrA CLIP-seq data of E. coli K1233, indicates that 104 out of the 159 mRNAs enriched in the foci (65%) were not detected in the K12 CLIP-seq analysis (Fig. 6C), whereas the sRNA overlap is much higher (Fig. 6D). Interestingly, three of the four CLIP-seq-specific sRNAs include one or no CsrA binding site. Notably, the two compared studies used two different E. coli strains (K12 and EPEC), grown in LB or DMEM, respectively, and subjected to different analyses (CLIP-seq and RNA-seq of enriched foci compartment). These differences may account for the observed differences in the repertoires of CsrA-bound RNAs.
A The RNA content of the CsrA foci. EPEC expressing CsrA-FLAGx3-GFP or wild-type EPEC expressing untagged CsrA (negative control) were grown to OD 0.6 and subjected to the foci-enrichment protocol (Fig. S9), followed by RNA extraction and library preparation. RNA-seq was performed and the sequenced RNA was mapped to the EPEC genome. The experiment was performed in three biological repeats and gene-assigned read counts from the six sequenced libraries were normalized and compared by DESeq254. DESeq2 reports for each of the compared genes the Log2 fold change (Log2FC) of the normalized read counts between the CsrA foci and the wt control along with the p-value corrected for multiple hypothesis testing (padj) (Supplementary Data 3). For a gene to be considered statistically significantly enriched in the CsrA foci we applied stringent filtering requiring Log2FC > 2 and padj<10−4. The genes that passed this stringent filtering are shown here with their respective Log2FC (x-axis) and –Log10 (padj) (y-axis) values. The identity of some of the genes and their biological function (manually curated from the literature) is indicated by color coding. RNAs belonging to the LEE1 (Ler, EscE) and LEE7 (GrlA) mRNAs are highlighted in bold fonts. B Representation of the different categories within the foci. A pie chart displays the number of genes detected in the CsrA foci assigned to the different categories. Genes were categorized as in (A). C mRNAs associated with the CsrA foci of EPEC compared to CsrA-associated RNAs in E. coli K12. A Venn diagram showing the genes obtained from the EPEC foci transcriptome (green circle) and E. coli K12 CsrA CLIP-seq analysis33 (red circle). Only genes common to the two strains were included in this comparison. The mRNAs were defined by their CDS boundaries. D sRNAs associated with the CsrA foci of EPEC compared to CsrA-associated sRNAs in E. coli K12. Like in (C), only sRNAs common to EPEC and E. coli K12 are compared. All of the sRNAs detected in the CsrA focus, but AspX, are known to contain two or more verified or putative CsrA binding sites (i.e., GGA triplet sequence). All of the sRNAs detected only in the CLIP-seq, but GcvB, contain one or no CsrA binding site33.
Importantly, several virulence-associated mRNAs were found to be foci-associated, including the transcripts of the LEE1 and LEE7 operons, both containing CsrA binding sites9,22 that are likely involved in their foci location. Furthermore, overexpressed CsrA was shown to bind to LEE7 and LEE1, inhibiting translation of encoded genes including that of Ler9, a regulator essential for transcription of most of the LEE operons, and for T3SS biogenesis3 (Fig. 7A). Unlike LEE1 and LEE7, which are negatively regulated by CsrA, the LEE4, encoding essential T3SS components, is positively regulated by CsrA9,35 (Fig. 7A). In contrast to the localization of the LEE1 and LEE7 transcripts, the LEE4 transcripts were not significantly enriched in the CsrA foci (Fig. 6A and Supplementary Data 3), despite having a CsrA binding site9,35. These results indicate that i) possessing CsrA binding sites is not necessarily sufficient for RNA recruitment to the focus compartment, and ii) that the presence of mRNAs in the CsrA foci correlates with their CsrA-dependent repression. Given these results, we asked whether T3SS production is deactivated by the recruitment of the LEE1 and LEE7 transcripts to the evolving focus compartment, where they might be repressed by the higher CsrA concentrations.
A Schematics of positive and negative regulation of the LEE operons by CsrA. For simplicity, only the main relevant details are shown. The LEE1 (solid green arrow) encodes for components that form the T3SS export apparatus (green components within the T3SS complex) and for Ler, which positively regulates all the other LEE operons (black, brown, and blue solid arrows). LEE2 and LEE3 encode many subunits of the T3SS basal body (indicated in brown shades), and LEE4 encodes for EscF, EspA, EspB, and EspD, which form the T3SS needle, filament, and translocation pore (blue shades). LEE7 encodes for GrlA (not shown in this scheme), a regulator that activates the LEE1 promoter and thus Ler expression. CsrA directly binds to the LEE4 transcript, promoting the expression of EspD and other LEE4-encoded proteins. Overexpressed CsrA binds to the LEE1 and LEE7 mRNAs, thus blocking the production of GrlA and Ler. The bacterial inner and outer membranes and host cell membrane are indicated (IM, OM and HM, respectively). Dashed arrows represent transcriptional regulation (red), post-transcriptional regulation (purple), and protein production (black). Created using BioRender. Rosenshine, I. (2025) https://BioRender.com/k60n247. B CsrA switches to ler repressive mode at the late logarithmic growth phase. Wild-type EPEC or ΔcsrA (strain NN5898) mutants were grown in DMEM to OD 0.3, or 0.9. Proteins were then extracted and subjected to western blot analysis using anti-Ler and anti-EspD antibodies. EspD is used here as a typical LEE4-encoded T3SS component. When indicated, the csrA mutation was complemented by plasmid expressing CsrA (pCsrA). A Δler mutant was used as a negative control. Total protein was used as loading control and a segment of the gel is shown. C Repression of infectivity at the late logarithmic growth phase. EPEC grown in DMEM to the indicated densities were used to infect HeLa cells. At 30 min post-infection, the cells were fixed and stained with phalloidin-rhodamine (actin, Red) and anti-EPEC antibody (green). Images were recorded using fluorescence microscopy. To score for infectivity, cells containing more than five T3SS-dependent actin pedestals were labeled as infected. In each sample of three independent biological repeats, n ≥ 60 HeLa cells were assessed. Data presented as mean values and standard deviation of three biological repeats, with an unpaired two-tailed t-test, p-value = 0.01 between OD 0.6 and 0.8 (*), and p-value = 0.001 between OD 0.6 and OD 1.0 (**). D Representative images from the experiment described in (C). White arrows indicate attached bacterial microcolonies associated with actin pedestals. Scale bar 10 µm. E CsrB overexpression represses Ler production. Wild-type EPEC harboring csrA-gfp (wt, strain NE9198), or isogenic mutant deleted of csrB (ΔcsrB, strain NE9542) were supplemented when indicated with a plasmid expressing CsrB (pZE-CsrB). Bacteria were grown with or without IPTG (0.1 mM), and upon reaching OD 0.3, proteins were extracted and subjected to western blot analysis using an anti-Ler antibody. A non-specific band (NS) was used as the loading control. The relative amount of Ler in percentage is indicated below the lanes (Expression). This was calculated using Image J, setting the wild-type levels as 100%, and normalized using the intensity of the nonspecific bands. F CsrB overproduction suppresses EPEC infectivity. Hela cells were infected with EPEC ΔcsrB mutant (strain NE9542) supplemented with a plasmid expressing CsrB under IPTG regulated promoter (ΔcsrB/pCsrB). IPTG was added (0.1 mM), or not, to the infection media as indicated. The infected cells were fixed and stained with phalloidin-rhodamine (actin, red) and anti-EPEC (green). Infectivity was scored as described in (C). In each biological repeat, n ≥ 60 HeLa cells were assessed for infection. Data presented as mean values and standard deviation of three independent experiments, with an unpaired two-tailed t-test p-value = 0.007 (**). G Representative images from the experiment described in (F). Arrows indicate clusters of actin pedestals. Scale bar is 10 µm.
CsrA switches from supporting to repressing T3SS expression
To test this idea, we monitored the production of the LEE1-encoded Ler and the LEE4-encoded EspD (Fig. 7A), during the transition from early to late logarithmic growth phase. The analysis showed that Ler is produced during the early growth phase, regardless of CsrA, whereas EspD production is both Ler and CsrA dependent (Fig. 7B). Notably, at the late growth phase, the Ler levels were diminished in a CsrA-dependent manner, consistent with the presence of LEE1 and LEE7 mRNAs within the repressing focus. Production of EspD was also repressed at this time likely due to the lack of Ler (Fig. 7A, B). We next tested the impact of the CsrA-dependent Ler repression on EPEC interaction with infected epithelial cells. We found that at the late growth phase, the T3SS is no longer functional, as reflected by the fading EPEC infectivity (Fig. 7C, D), suggesting that CsrA is required for repressing virulence at the late growth phase. Overall, our findings align with previous studies indicating that both the absence and overexpression of CsrA inhibit the expression of a functional T3SS9,22. The results further support the idea that at the early growth phase, when the bacteria lack foci, the cytoplasmic CsrA levels are too low to repress Ler production, yet sufficient to support the translation of LEE4 encoded proteins. Later, the foci start to evolve coinciding with the LEE1 and LEE7 transcripts recruitment to this compartment. We predict that the LEE1 and LEE7 are repressed in this compartment by the higher CsrA levels, hence, eventually halting T3SS production and subsequently dampening the pathogen’s virulence.
CsrA and CsrB cooperate in ler repression
The CsrB function as a CsrA inhibitor via competition with target mRNAs is well documented15. In contrast, the above model predicts that by promoting foci formation, CsrB is enhancing Ler repression by CsrA. To examine this prediction, we boosted foci expansion at the early logarithmic growth phase by ectopically overexpressing CsrB, and monitored its impact on Ler production. As controls, we examined the production of EspD and NleA, known to be positively and negatively regulated by CsrA, respectively9,22. CsrB overexpression led to the formation of large foci (Fig. S7A) and as expected it inhibited CsrA, leading to activation and repression of NleA and EspD production, respectively (Fig. S14). In contrast, the overexpressed CsrB not only fails to antagonize the repression of Ler by CsrA, but instead, it cooperates with CsrA to enhance Ler repression (Fig. 7E) and consequently to curb EPEC infectivity (Fig. 7F, G). The minor effect of the deletion of CsrB on the elevation of Ler levels (Fig. 7E), is related to the Ler autorepression activity, forming a negative feedback loop that sets the maximal Ler levels36,37. Taken together, these results point to a dual CsrB function, which is typically considered a CsrA antagonist. Yet, in the focus context, CsrA and CsrB appear to cooperate in ler regulation, highlighting the role of the CsrA focus as a complex regulatory hub.
Discussion
Posttranscriptional regulation emerges as a key mechanism in virulence control with the CSR system executing this regulation in many pathogens, including E. coli, Yersinia, Salmonella, Shigella, P. aeruginosa, V. cholerae, Legionella pneumophila and H. pylori21,25,28,38. However, the mechanism of this regulation is only partially understood. Here, we show that CsrA forms a membraneless compartment in several pathogens including EPEC. According to our model, the driving force for the assembly of this compartment is CsrA-CsrB interactions (Fig. 8). Analysis of enriched CsrA foci shows that this compartment is composed of CSR and degradosome components, as well as a defined set of regulatory sRNAs and selected mRNAs. The formation of this compartment gives rise to the establishment of two CsrA subpopulations: one located in the cytoplasm, with constant CsrA levels, and the other located in the focus compartment with higher CsrA concentrations. We conclude that the pathogen takes advantage of the dynamics of the CsrA foci biogenesis to switch from virulence-supporting to virulence-repressing mode. This switch includes temporal recruitment of a defined set of mRNAs to this compartment where they are spatially regulated (Fig. 8). Accordingly, during the early growth phase, when the bacteria are highly infective, the CsrA-focus compartment is not yet assembled, or remains minor, whereas the cytoplasmic CsrA dominates with concentrations suitable to promote the translation of the LEE4 transcript, yet too low to cause a significant Ler repression. Our data support the notion that the expanding CsrA focus compartment dictates the recruitment of the LEE1 and LEE7 transcripts to the foci, leading eventually to Ler repression by the cooperative action of CsrA and CsrB, hence; halting the pathogen’s virulence (Fig. 8).
A CsrA switches from supporting to repressing T3SS expression. During the early exponential growth phase (left half of the illustrated bacterium), most bacteria either lack CsrA foci or have small foci. In these bacteria, the cytoplasmic CsrA levels are relatively low (represented by the light green shade), allowing Ler expression, which triggers a positive feedback loop leading to the transcription of the LEE operons (blue arrow). The transcripts of these operons are shown collectively in a blue transparent rectangle. In these bacteria, the CsrA levels are sufficient to support the translation of key T3SS proteins in the LEE4 transcript (Pink arrow), leading to T3SS assembly and virulence ON state. When reaching the late exponential growth phase (right half of the illustrated bacterium), the LEE1 and LEE7 mRNAs are localized in the expanding focus. In this compartment, the higher CsrA concentration acts to repress Ler production (pink blunt arrow). This breaks the positive feedback loop, thus leading to a decay in T3SS expression and diminishing infectivity. Created using BioRender. Rosenshine, I. (2025) https://BioRender.com/k60n247. B Model of CsrA focus composition. CsrA dimers bridged by CsrB form a network constituting the focus backbone. The CsrB function is complex, as in addition to its structural function and inhibition of CsrA, it surprisingly also collaborates with CsrA to achieve repression of ler, and possibly other genes. Additional mRNAs and regulatory sRNAs are distributed between the focus and cytoplasmic compartments, and localized to the different compartments based on their affinity to CsrA, number of CsrA binding sites, and abundance. Other RNAs might be recruited to the foci via base pairing with foci-associated RNAs. Some regulatory processes may be executed within the condensates, such as post-transcriptional regulation by CsrA or/and foci-associated sRNAs. Alternatively, these compartments may store or sequester molecules, or function as RNA and/or protein degradation centers. The decay rate and processing of focus-associated RNAs might be executed by the foci-associated RNase E, which is also anchored to the inner membrane. The association of RNase E with the foci might be driven by RNAs that bridge CsrA and RNase E. Created using BioRender. Rosenshine, I. (2025) https://BioRender.com/k60n247.
Transcripts other than those of the LEE, which are recruited to the focus are expected to be co-regulated at this compartment, unleashing extensive remodeling of gene expression and adaptation to the changing conditions. These focus-associated transcripts encode metabolic pathways such as iron acquisition factors, toxin/anti-toxin systems, as well as additional virulence-related proteins (e.g., type 1 pili components and EspC toxin, Supplementary Data 3). Thus, whereas our results provide the fundamental principles of how the CsrA focus compartment operates, the full spectrum of the focus-dependent regulation and how the focus-associated sRNAs, CSR and degradosome components work together to remodel the gene expression remains to be elucidated.
The emergence of CsrA foci is driven by specific and strong CsrA-RNA interactions, forming highly stable particles that endure several purification steps including the high centrifugal force during ultracentrifugation (Fig. S9). In contrast, the driving forces for the formation of the few reported bacteria condensates (i.e., condensates generated by RNA polymerase39,40; Hfq41,42; DEAD-box ATPases43, RNase E44, and Rho45), are weak interactions between intrinsically disordered protein domains and/or weak electrostatic interactions between RNA and positively charged proteins. The uniqueness of the CsrA compartment is further reflected by its distinct morphology. All of the above condensates form multiple small puncta, whereas CsrA typically forms a singular, large focus. Taken together our results suggest that the CsrA focus is a compartment different from the previously reported condensates.
The CSR system is conserved across the bacterial kingdom, regulating complex bacterial behaviors, such as metabolism, biofilm formation, production of secondary messengers, and virulence12,46. We thus predict that the formation of similar CsrA membraneless compartments likely constitutes a widespread regulatory strategy in bacteria. Furthermore, we predict that each of these bacteria has tailored the use of the CSR condensate to support its unique specific needs. We propose to term the CsrA membraneless regulatory hub “CSRsome”.
Methods
Strains, plasmids, primers, and basic procedures
Bacterial strains, plasmids, primers/probes and antibodies are listed in Tables S1–S6. Strains were constructed using the lambda red system, as described in refs. 47,48,49. Plasmids were constructed using standard methods or isothermal assembly kit (Cat. E2611L, New England Biolabs). The desired clone was verified by PCR reaction and Sanger sequencing. Bacteria were grown in Luria-Bertani (LB) broth supplemented when needed, with ampicillin (Amp, 100 μg/ml), streptomycin (Strep, 50 μg/ml), chloramphenicol (Cm, 25 μg/ml), tetracycline (Tet, 20 μg/ml), kanamycin (Kn, 40 μg/ml) or Spectinomycin (50 μg/ml). Expression of recombinant proteins and RNAs was induced by isopropyl ß-D-1-thiogalactopyranoside (IPTG), or anhydrotetracycline (aTc) at the indicated concentrations. When indicated the control over IPTG-induced promoters was tightened by supplementing the relative strain with a plasmid overexpressing LacI (pREP4).
Bacterial growth conditions
To mimic host conditions (“activating conditions”), which lead to activation of the expression of the virulence machinery, bacteria were grown statically overnight in LB at 37 °C, then sub-cultured by diluting bacteria 1:50 or 1:100 with Dulbecco’s modified Eagle medium (DMEM) without phenol-red, (Biological Industries 01-053-1 A), and grown for 3 to 8 h at 37 °C as indicated. In experiments where CsrA-GFP was expressed from plasmids, cultures were grown to OD of 0.45-0.6, and expression was induced by adding 0.05 mM of IPTG during the final hour of growth.
Microscopy
Bacteria expressing fluorescent proteins were grown under activating conditions, fixed in 3.7% formaldehyde for 10 minutes at room temperature, washed twice in PBS, placed on a glass slide coated with poly L lysine (Cat. P8920, Sigma), and analyzed by fluorescence microscopy (Nikon TI2-P-FWBS-E, or Zeiss Axiovert, set with a camera 01-PRIME-BSI-R-M-16-C-META). Images were analyzed using Image J or Nikon NIS.
Immunofluorescence microscopy
Cultures of EPEC chromosomally tagged CsrA with FLAGx3, or wild-type EPEC, were grown for 3 hours in infection-mimicking conditions. Bacteria were then fixed with 4% formaldehyde and 0.01% glutaraldehyde for 20 min at room temperature, washed twice with phosphate buffer saline (PBS), and resuspended with 70% ethanol on a nutator for one hour at room temperature. Afterwards, PBS was added to the ethanol at a ratio of 1:1 and centrifuged at 600 g for five minutes. Bacteria were resuspended with PBS and mounted on Poly-L-lysine coverslips for 1 h at 4 °C. Cells were then washed once with PBS and treated with 25 µg/ml of lysozyme in TEG buffer (25 mM Tris-HCl, 10 mM EDTA, and 50 mM glucose) for 10 min. Then bacteria were washed three times, and incubated with a blocking solution (0.5% BSA in PBS) for half an hour at RT. For immunostaining, the CsrA-FLAGx3, anti-FLAG M2 mouse monoclonal antibody (F1804, Sigma) and Anti-mouse Alexa 647 conjugate antibody (4414S, cell signaling) were used. The significance of CsrA-RNase E colocalization was assessed as described in ref. 50, and also using the approach described in Fig. S11.
RNA localization
Bacteria containing two plasmids were used: i) Plasmids expressing under lac promoter one of the following RNAs: CsrB (pNE9598), or MS2tag (pLA12032), or MS2tag fused to the 5’ of either CsrB or CsrC (pLA10176 and pLA10177); and ii) a plasmid expressing the MS2 protein fused to GFP (pLA11940). Bacteria were grown under activating conditions and at 3 h post inoculum the cultures were transferred to a 20 °C incubator, and IPTG (0.1 mM) was added to activate RNA expression. Anhydrous tetracycline (aTc, 1 ng/ml) was added after 1 hour post IPTG induction and at 5 hours post inoculation the cultures were fixed and analyzed. The incubation at 20 °C was needed to prevent the formation of inclusion bodies by the overexpressed MS2-GFP.
Quantification of foci sum of intensity and average area size
CsrA-GFP foci were analyzed using Image J, by thresholding the GFP signal, detecting the foci in each field, and measuring the sum of intensity and average area of each focus. The signal was normalized to the largest focus measured in a single set of experiments. In the experiments showing the foci/cytoplasm signal ratio, the measurement of GFP signal in the cytoplasm and the focus was done in a constant area (i.e. relative concentration). The ratio of foci/cytoplasm intensity was calculated by dividing the sum of intensity of the CsrA focus and the cytoplasm of each individual bacterium.
Analysis of CsrA dimerization
Merodiploid EPEC strains expressing CsrA-FLAG and CsrA-GFP were statically grown in LB overnight at 37 °C. The next day, the starters were sub-cultured in DMEM, and grown for 5 hours to an OD of ~0.4-0.5. 10 OD units of bacteria were pelleted and snap-freezed in liquid nitrogen. Bacteria were then suspended in 800 µl of lysis/washing buffer containing 50 mM sodium phosphate, 300 mM NaCl, 1:50 complete protease inhibitor-EDTA free (Roche), loaded onto glass beads 0.1 mm (Biospec, Cat. 11079101) and lysed using the mixer mill (Retch, model no. MM400). The crude bacterial lysate was cleared by centrifugation and further incubated with 20 µl of anti-FLAG magnetic beads (M8823, sigma) for 1 h at 4 °C. Next, beads were washed four times in lysis/washing buffer, boiled for 10 minutes in 100 µl of Laemmli buffer (Bio-Rad), and subjected to analysis by Western blotting using anti-FLAG and anti-GFP antibodies.
Western blot analysis
Normalized bacterial extracts were boiled for 10 min in Laemmli buffer (Bio-Rad Cat. 1610747), centrifuged for 2 min at 12,000 g at room temperature, and subjected to SDS-PAGE (typically using Bio-Rad 12% TGX pre-cast gels). Loading amounts were normalized using protein concentration or OD of the used culture and further verified by recording the gels using stain-free imaging (Bio-Rad). Uncropped and unprocessed scans of all the blots are in the Source Data file.
RNA extraction and real-time PCR
RNA was extracted using TRI reagent (T9424, Sigma) according to the manufacturer’s instructions. RNA (1.5 ug) was treated with RQ1 DNase I (M610A, Promega) at a concentration of 1U/µg RNA for 30 min at 37 °C. DNase I was inactivated by adding 1 µl of stop solution and heating the samples for 15 min at 65 °C. DNA digestion was verified by PCR, using primers #1952 and #1953. cDNA was synthesized using qPCRBIO high-quality cDNA synthesis kit (PB30.11-10, PCR Biosystems) and quantified by Real-Time PCR (RT-PCR) using iTaq Universal SYBR Green Supermix (Cat. 1725124, Bio-Rad) with a CFX96 Real-Time System (Bio-Rad) according to the manufacturer’s instructions. The level of 16S rRNA (rrsB) was used to normalize the expression levels of csrB. The relative amount of cDNA was calculated by the standard curve method (ΔΔCq), which was obtained by PCR of serially diluted genomic DNA as standard and analyzed using Bio-Rad CFX maestro software.
Microscopy of HeLa cells infection
HeLa cells (ATCC, CCL-2) were seeded on glass slides in a 24 well-plate (NuncTM) at a density of ~7 × 104 cells per well and grown overnight in DMEM supplemented with 10% fetal calf serum (FCS, Cat. F9665, Biological Industries) and antibiotics (penicillin-streptomycin solution, Cat. 15140-122, Gibco). Next, HeLa cells were washed and infected with bacteria statically grown overnight at 37 °C (multiplicity of infection ~1:100) followed by 2.5–8 h incubation at 37 °C, 5% CO2. To terminate infection, cells were fixed in 3.7% formaldehyde in PBS, washed twice (PBS), perforated (0.25% Triton-X100 in PBS for 10 min), washed twice (PBS), stained with rabbit anti-EPEC antibody and anti-Rabbit IgG Alexa Fluor 488 and phalloidin rhodamine (Cat. P1951, Sigma).
CsrA focus stability assays
Cultures of EPEC csrA-gfp were grown under activating conditions for 3 h, then chloramphenicol (100 µg/ml) or rifampicin (200 µg/ml) were added and incubated for an additional hour. Samples were taken before and after the addition of the relevant antibiotics, fixed in 3.7% formaldehyde in PBS, washed twice (PBS), and visualized by microscopy.
Purification of CsrA foci
Cultures of EPEC expressing CsrA-GFP, or CsrA-FLAG-GFP, or as negative control, wild-type EPEC, were grown in DMEM standing conditions at 37 °C to D600 0.6, pelleted, and gently lysed on ice using in a lysis buffer (200 mM Tris pH 8, 20% sucrose, 2% protease inhibitor without EDTA (Roche Cat. 1-836-170), recombinant RNase Inhibitor 0.1 U/µl (Cat. 2313 A Takara), DNase I 25 µg/ml, 1% IGEPAL (Cat. I8896 Sigma) and lysozyme 0.25 mg/ml). The lysate was cleared from the cell debris by centrifugation (4 °C, 18,400 g, 10 min) and the foci-containing supernatant was collected. The foci were then pelleted by ultracentrifugation (4 °C, 186,000 g for 1 h, TLA-55 Beckman Coulter Optima TLX Ultracentrifuge). The pellets were gently suspended with washing buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, EDTA free protease inhibitor and recombinant RNase inhibitor 0.1 U/ul) and pelleted again as before. The pellets containing the CsrA-GFP foci were then gently suspended in 500 µl of washing buffer and subjected to affinity purification. In case of CsrA-GFP purification the foci were immuno-precipitated with anti-GFP trap particles (gtd-20, Chromotek). To purify the CsrA-FLAG-GFP the foci were first by immuno-precipitated with anti-FLAG beads (Cat M8823, Sigma), eluted with FLAG peptide (Sigma Cat F4799), and the eluate was subjected to second precipitation step using anti-GFP trap particles (gtd-20, Chromotek). Samples from the different purification steps were kept for analysis by western blots and microscopy.
LC-MS/MS analysis of the CsrA foci
Proteins attached to the GFP trap particles were trypsinized and analyzed by the LC-MS/MS using the Q-Exactive HF (Thermo) mass spectrometer. The data was analyzed using Maxquant package (Mathias Mann lab, Max Planck Institute) for identification and quantification against the enteropathogenic E. coli proteome (UniProt database). All the identified peptides were filtered with high confidence, top rank, mass accuracy, and score vs. charge state. High-confidence peptides have passed the 1% FDR (false discovery rate) threshold, which is the estimated fraction of false positives in a list of peptides. Semi-quantitation was done by calculating the peak area of each peptide. The area of the protein is the average of the three most intense peptides from each protein. Statistical analysis (t-test with p-value) was done using the Perseus software.
Transcriptome analysis of the CsrA foci
RNA was extracted from enriched CsrA foci (CsrA-FLAGx3-GFP, strain LA9743) and from wild-type EPEC, as a negative control for non-specific bead-associated RNA. The RNA from three biological replicates for each strain was used for construction of six libraries as described51, starting from step #53. The six libraries were sequenced by single-end sequencing, reads were processed with the Cutadapt tool52 (v. 3.5) and mapped, using bwa software package53 (v.0.7.17-r1188), to EPEC E2348/69 chromosome (NC_0011601.1) and plasmids pE2348-2 (NC_011602.1), pMAR2 (NC_011603.1) p5217 (EU580135.1). DESeq254 was used for the normalization of sequencing raw-counts and for differential expression analysis.
Identification of RNAs enriched in the CsrA foci
For each of the six libraries, reads were assigned to EPEC genes following Pearl Mizrahi et al.55 gene annotation (Supplementary Table S8 in ref. 55, and Supplementary Methods therein). Reads were considered only if they were unambiguously annotated, i.e., the read did not map to a genomic region spanning two adjacent genes. Reads that could not be assigned to an annotated gene were classified as either Intergenic (residing between annotated genes) or Antisense (residing opposite to an annotated gene). The differential analysis comparing gene normalized read counts between CsrA-FLAGx3-GFP and wt control libraries was performed by the R statistical software56 (v. 4.1.0), using DESeq254,55 package (v. 1.3). DESeq2 was applied with the default parameters except for the exclusion of the independent filtering, and the addition of pre-filtering of 1. A gene was considered statistically significantly enriched in the CsrA foci if its DESeq2 normalized read count in the CsrA-FLAGx3-GFP libraries was statistically significantly higher compared to the wt libraries. We used stringent thresholds requiring Log2 fold change between the compared libraries > 2 and p-value corrected for multiple hypothesis (padj) < 10−4.
Statistics and reproducibility
Statistical analysis was conducted using GraphPad Prism 8 and Excel 365. Each experiment in this study was performed at least three times unless otherwise stated. The results are presented as mean ± standard deviation. An unpaired two-tailed Student’s t-test was used to calculate p values, with p-values < 0.05 considered statistically significant. p-values are denoted as follows: p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****). No data were excluded from the analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The paper Supplementary Information and Source Data files include all the data needed to support the conclusions of this paper. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD061003. The RNA-seq data generated in this study have been deposited in the ArrayExpress database under accession code E-MTAB-14951 (https://www.ebi.ac.uk/biostudies/arrayexpress/studies/E-MTAB-14951). Source data are provided with this paper.
References
Ochoa, T. J. & Contreras, C. A. Enteropathogenic Escherichia coli infection in children. Curr. Opin. Infect. Dis. 24, 478–483 (2011).
Gaytán, M. O., Martínez-Santos, V. I., Soto, E. & González-Pedrajo, B. Type Three Secretion System in Attaching and Effacing Pathogens. Front Cell Infect. Microbiol 6, 129 (2016).
Yerushalmi, G., Litvak, Y., Gur-Arie, L. & Rosenshine, I. Dynamics of expression and maturation of the type III secretion system of enteropathogenic Escherichia coli. J. Bacteriol. 196, 2798–2806 (2014).
Ruano-Gallego, D. et al. Type III secretion system effectors form robust and flexible intracellular virulence networks. Science 371, https://doi.org/10.1126/science.abc9531 (2021).
Barba, J. et al. A positive regulatory loop controls expression of the locus of enterocyte effacement-encoded regulators Ler and GrlA. J. Bacteriol. 187, 7918–7930 (2005).
Yang, J., Tauschek, M., Hart, E., Hartland, E. L. & Robins-Browne, R. M. Virulence regulation in Citrobacter rodentium: the art of timing. Micro. Biotechnol. 3, 259–268 (2010).
Furniss, R. C. D. & Clements, A. Regulation of the Locus of Enterocyte Effacement in Attaching and Effacing Pathogens. J. Bacteriol. 200, https://doi.org/10.1128/JB.00336-17 (2018).
Volk, M., Vollmer, I., Heroven, A. K. & Dersch, P. Transcriptional and Post-transcriptional Regulatory Mechanisms Controlling Type III Secretion. Curr. Top. Microbiol Immunol. 427, 11–33 (2020).
Bhatt, S. et al. The RNA binding protein CsrA is a pleiotropic regulator of the locus of enterocyte effacement pathogenicity island of enteropathogenic Escherichia coli. Infect. Immun. 77, 3552–3568 (2009).
Rife, C. et al. Crystal structure of the global regulatory protein CsrA from Pseudomonas putida at 2.05 A resolution reveals a new fold. Proteins 61, 449–453 (2005).
Mercante, J., Edwards, A. N., Dubey, A. K., Babitzke, P. & Romeo, T. Molecular geometry of CsrA (RsmA) binding to RNA and its implications for regulated expression. J. Mol. Biol. 392, 511–528 (2009).
Pourciau, C., Lai, Y. J., Gorelik, M., Babitzke, P. & Romeo, T. Diverse Mechanisms and Circuitry for Global Regulation by the RNA-Binding Protein CsrA. Front Microbiol 11, 601352 (2020).
Dubey, A. K. et al. CsrA regulates translation of the Escherichia coli carbon starvation gene, cstA, by blocking ribosome access to the cstA transcript. J. Bacteriol. 185, 4450–4460 (2003).
Wei, B. L. et al. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol. Microbiol 40, 245–256 (2001).
Liu, M. Y. et al. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J. Biol. Chem. 272, 17502–17510 (1997).
Weilbacher, T. et al. A novel sRNA component of the carbon storage regulatory system of Escherichia coli. Mol. Microbiol 48, 657–670 (2003).
Leng, Y. et al. Regulation of CsrB/C sRNA decay by EIIA(Glc) of the phosphoenolpyruvate: carbohydrate phosphotransferase system. Mol. Microbiol 99, 627–639 (2016).
Suzuki, K., Babitzke, P., Kushner, S. R. & Romeo, T. Identification of a novel regulatory protein (CsrD) that targets the global regulatory RNAs CsrB and CsrC for degradation by RNase E. Genes Dev. 20, 2605–2617 (2006).
Jonas, K. et al. The RNA binding protein CsrA controls cyclic di-GMP metabolism by directly regulating the expression of GGDEF proteins. Mol. Microbiol 70, 236–257 (2008).
Baker, C. S., Morozov, I., Suzuki, K., Romeo, T. & Babitzke, P. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol. Microbiol 44, 1599–1610 (2002).
Kusmierek, M. & Dersch, P. Regulation of host-pathogen interactions via the post-transcriptional Csr/Rsm system. Curr. Opin. Microbiol 41, 58–67 (2018).
Katsowich, N. et al. Host cell attachment elicits posttranscriptional regulation in infecting enteropathogenic bacteria. Science 355, 735–739 (2017).
Umanski, T., Rosenshine, I. & Friedberg, D. Thermoregulated expression of virulence genes in enteropathogenic Escherichia coli. Microbiology 148, 2735–2744 (2002).
Ronin, I., Katsowich, N., Rosenshine, I. & Balaban, N. Q. A long-term epigenetic memory switch controls bacterial virulence bimodality. Elife 6, https://doi.org/10.7554/eLife.19599 (2017).
Heroven, A. K., Böhme, K. & Dersch, P. The Csr/Rsm system of Yersinia and related pathogens: a post-transcriptional strategy for managing virulence. RNA Biol. 9, 379–391 (2012).
Valverde, C., Lindell, M., Wagner, E. G. & Haas, D. A repeated GGA motif is critical for the activity and stability of the riboregulator RsmY of Pseudomonas fluorescens. J. Biol. Chem. 279, 25066–25074 (2004).
Mercante, J., Suzuki, K., Cheng, X., Babitzke, P. & Romeo, T. Comprehensive alanine-scanning mutagenesis of Escherichia coli CsrA defines two subdomains of critical functional importance. J. Biol. Chem. 281, 31832–31842 (2006).
Heroven, A. K., Böhme, K., Rohde, M. & Dersch, P. A Csr-type regulatory system, including small non-coding RNAs, regulates the global virulence regulator RovA of Yersinia pseudotuberculosis through RovM. Mol. Microbiol 68, 1179–1195 (2008).
Carpousis, A. J. The Escherichia coli RNA degradosome: structure, function and relationship in other ribonucleolytic multienzyme complexes. Biochem Soc. Trans. 30, 150–155 (2002).
Bandyra, K. J., Bouvier, M., Carpousis, A. J. & Luisi, B. F. The social fabric of the RNA degradosome. Biochim Biophys. Acta 1829, 514–522 (2013).
Strahl, H. et al. Membrane recognition and dynamics of the RNA degradosome. PLoS Genet. 11, e1004961 (2015).
Urban, J. H. & Vogel, J. Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res. 35, 1018–1037 (2007).
Potts, A. H. et al. Global role of the bacterial post-transcriptional regulator CsrA revealed by integrated transcriptomics. Nat. Commun. 8, 1596 (2017).
Rojano-Nisimura, A. M. et al. CsrA selectively modulates sRNA-mRNA regulator outcomes. Front Mol. Biosci. 10, 1249528 (2023).
Wang, D. et al. An RNA-dependent mechanism for transient expression of bacterial translocation filaments. Nucleic Acids Res 46, 3366–3381 (2018).
Yerushalmi, G., Nadler, C., Berdichevski, T. & Rosenshine, I. Mutational analysis of the locus of enterocyte effacement-encoded regulator (Ler) of enteropathogenic Escherichia coli. J. Bacteriol. 190, 7808–7818 (2008).
Berdichevsky, T. et al. Ler is a negative autoregulator of the LEE1 operon in enteropathogenic Escherichia coli. J. Bacteriol. 187, 349–357 (2005).
Barnard, F. M. et al. Global regulation of virulence and the stress response by CsrA in the highly adapted human gastric pathogen Helicobacter pylori. Mol. Microbiol 51, 15–32 (2004).
Ladouceur, A. M. et al. Clusters of bacterial RNA polymerase are biomolecular condensates that assemble through liquid-liquid phase separation. Proc. Natl Acad. Sci. USA 117, 18540–18549 (2020).
Weng, X. et al. Spatial organization of RNA polymerase and its relationship with transcription in. Proc. Natl Acad. Sci. USA 116, 20115–20123 (2019).
McQuail, J., Switzer, A., Burchell, L. & Wigneshweraraj, S. The RNA-binding protein Hfq assembles into foci-like structures in nitrogen starved. J. Biol. Chem. 295, 12355–12367 (2020).
Goldberger, O., Szoke, T., Nussbaum-Shochat, A. & Amster-Choder, O. Heterotypic phase separation of Hfq is linked to its roles as an RNA chaperone. Cell Rep. 41, 111881 (2022).
Hondele, M. et al. DEAD-box ATPases are global regulators of phase-separated organelles. Nature 573, 144–148 (2019).
Al-Husini, N., Tomares, D. T., Bitar, O., Childers, W. S. & Schrader, J. M. α-Proteobacterial RNA Degradosomes Assemble Liquid-Liquid Phase-Separated RNP Bodies. Mol. Cell 71, 1027–1039.e1014 (2018).
Krypotou, E. et al. Bacteria require phase separation for fitness in the mammalian gut. Science 379, 1149–1156 (2023).
Timmermans, J. & Van Melderen, L. Post-transcriptional global regulation by CsrA in bacteria. Cell Mol. Life Sci. 67, 2897–2908 (2010).
Uzzau, S., Figueroa-Bossi, N., Rubino, S. & Bossi, L. Epitope tagging of chromosomal genes in Salmonella. Proc. Natl Acad. Sci. USA 98, 15264–15269 (2001).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Li, X. T., Thomason, L. C., Sawitzke, J. A., Costantino, N. & Court, D. L. Positive and negative selection using the tetA-sacB cassette: recombineering and P1 transduction in Escherichia coli. Nucleic Acids Res 41, e204 (2013).
Nandana, V. et al. The BR-body proteome contains a complex network of protein-protein and protein-RNA interactions. Cell Rep. 42, 113229 (2023).
Melamed, S. et al. Mapping the small RNA interactome in bacteria using RIL-seq. Nat. Protoc. 13, 1–33 (2018).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10–12 (2025).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM arXiv. Genomics https://doi.org/10.48550/arXiv.1303.3997 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Pearl Mizrahi, S. et al. The impact of Hfq-mediated sRNA-mRNA interactome on the virulence of enteropathogenic. Sci. Adv. 7, eabi8228 (2021).
RStudio: Integrated Development for R v. 4.1.0 (Boston, MA, USA, 2021).
Acknowledgements
We thank Orna Amster Choder (HUJI), Gad Frankel (Imperial College), and Samantha Gruenheid (McGill) for providing antibodies and plasmids, the members of the I.R., H.M., and S.B.Y. groups for critical reading of the manuscript. I.R. and S.B.Y. were supported by European Research Council Synergy grant #810186, I.R. by Israel Science Foundation grant 743/18 and HM by European Research Council grant 833598, and Israel Science Foundation grant 876/17.
Author information
Authors and Affiliations
Contributions
Conceptualization: N.E., L.A., I.R.; Methodology: H.M., L.A., L.A.R.G.*, R.F.R., Y.A., O.Y.; Investigation: L.A., N.E., N.K.; Visualization: L.A., N.E., N.K.; Funding acquisition: I.R., S.B.Y., H.M.; Project administration: M.B.S., M.R.; Supervision: I.R., H.M., S.B.Y.; Writing original draft: L.A., N.E., I.R., S.B.Y.; Writing review & editing: I.R., S.B.Y. *LARG- Liron Argaman.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aroeti, L., Elbaz, N., Faigenbaum-Romm, R. et al. Formation of a membraneless compartment regulates bacterial virulence. Nat Commun 16, 3834 (2025). https://doi.org/10.1038/s41467-025-58829-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58829-9