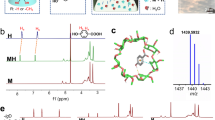
Abstract
Despite its disordered liquid-like structure, glass exhibits solid-like mechanical properties1. The formation of glassy material occurs by vitrification, preventing crystallization and promoting an amorphous structure2. Glass is fundamental in diverse fields of materials science, owing to its unique optical, chemical and mechanical properties as well as durability, versatility and environmental sustainability3. However, engineering a glassy material without compromising its properties is challenging4,5,6. Here we report the discovery of a supramolecular amorphous glass formed by the spontaneous self-organization of the short aromatic tripeptide YYY initiated by non-covalent cross-linking with structural water7,8. This system uniquely combines often contradictory sets of properties; it is highly rigid yet can undergo complete self-healing at room temperature. Moreover, the supramolecular glass is an extremely strong adhesive yet it is transparent in a wide spectral range from visible to mid-infrared. This exceptional set of characteristics is observed in a simple bioorganic peptide glass composed of natural amino acids, presenting a multi-functional material that could be highly advantageous for various applications in science and engineering.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the Article and the Supplementary Information.
References
Zanotto, E. D. & Mauro, J. C. The glassy state of matter: its definition and ultimate fate. J. Non Cryst. Solids 471, 490–495 (2017).
Debenedetti, P. G. & Stillinger, F. H. Supercooled liquids and the glass transition. Nature 410, 259–267 (2001).
Shelby, J. E. Introduction to Glass Science and Technology (Royal Society of Chemistry, 2005).
Yanagisawa, Y., Nan, Y., Okuro, K. & Aida, T. Mechanically robust, readily repairable polymers via tailored noncovalent cross-linking. Science 359, 72–76 (2018).
Wang, H. et al. Room-temperature autonomous self-healing glassy polymers with hyperbranched structure. Proc. Natl Acad. Sci. USA 117, 11299–11305 (2020).
Huang, Z. et al. Highly compressible glass-like supramolecular polymer networks. Nat. Mater. 21, 103–109 (2022).
Zhang, Q. et al. Formation of a supramolecular polymeric adhesive via water-participant hydrogen bond formation. J. Am. Chem. Soc. 141, 8058–8063 (2020).
Dong, S. et al. Structural water as an essential comonomer in supramolecular polymerization. Sci. Adv. 3, eaao0900 (2017).
Barrat, J. L., Baschnagel, J. & Lyulin, A. Molecular dynamics simulations of glassy polymers. Soft Matter 6, 3430–3446 (2010).
Colmenero, J. Are polymers standard glass-forming systems? the role of intramolecular barriers on the glass-transition phenomena of glass-forming polymers. J. Phys. Condens. Matter 27, 103101 (2015).
Balkenende, D. W. R., Monnier, C. A., Fiore, G. L. & Weder, C. Optically responsive supramolecular polymer glasses. Nat. Commun. 7, 1–9 (2016).
Lebel, O. & Soldera, A. in Advanced Materials (eds van de Ven, T. & Soldera, A.) 239–260 (De Gruyter, 2019).
Wuest, J. D. & Lebel, O. Anarchy in the solid state: structural dependence on glass-forming ability in triazine-based molecular glasses. Tetrahedron 65, 7393–7402 (2009).
Chaplin, M. Do we underestimate the importance of water in cell biology? Nat. Rev. 7, 861–866 (2006).
Hazra, P., Chakrabarty, D. & Sarkar, N. Intramolecular charge transfer and solvation dynamics of Coumarin 152 in aerosol-OT, water-solubilizing reverse micelles, and polar organic solvent solubilizing reverse micelles. Langmuir 18, 7872–7879 (2002).
Levin, A. et al. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 4, 615–634 (2020).
Reches, M. & Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 300, 625–627 (2003).
Tao, K., Makam, P., Aizen, R. & Gazit, E. Self-assembling peptide semiconductors. Science 358, eaam9756 (2017).
Gilead, S. & Gazit, E. Self-organization of short peptide fragments: from amyloid fibrils to nanoscale supramolecular assemblies. Supramol. Chem. 17, 87–92 (2005).
Zhao, X. & Zhang, S. Designer self-assembling peptide materials. Macromol. Biosci. 7, 13–22 (2007).
Cui, H., Webber, M. J. & Stupp, S. I. Self‐assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Pept. Sci. 94, 1–18 (2010).
Fleming, S. & Ulijn, R. V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 43, 8150–8177 (2014).
Knowles, T. P. J., Oppenheim, T. W., Buell, A. K., Chirgadze, D. Y. & Welland, M. E. Nanostructured films from hierarchical self-assembly of amyloidogenic proteins. Nat. Nanotechnol. 5, 3–6 (2010).
Chung, C. W. et al. Label-free characterization of amyloids and alpha-synuclein polymorphs by exploiting their intrinsic fluorescence property. Anal. Chem. 13, 5367–5374 (2022).
Adler-Abramovich, L. et al. Bioinspired flexible and tough layered peptide crystals. Adv. Mater. 30, 1–6 (2018).
Adler-Abramovich, L. & Gazit, E. The physical properties of supramolecular peptide assemblies: From building block association to technological applications. Chem. Soc. Rev. 43, 6881–6893 (2014).
Lampel, A., Ulijn, R. V. & Tuttle, T. Guiding principles for peptide nanotechnology through directed discovery. Chem. Soc. Rev. 47, 3737–3758 (2018).
Ulijn, R. V. & Smith, A. M. Designing peptide based nanomaterials. Chem. Soc. Rev. 37, 664–675 (2008).
Kholkin, A., Amdursky, N., Bdikin, I., Gazit, E. & Rosenman, G. Strong piezoelectricity in bioinspired peptide nanotubes. ACS Nano 4, 610–614 (2010).
Yan, X. & Li, J. Self-assembly and application of diphenylalanine-based nanostructures. Chem. Soc. Rev. 39, 1877–1890 (2010).
Arnon, Z. A. et al. On-off transition and ultrafast decay of amino acid luminescence driven by modulation of supramolecular packing. iScience 24, 102695 (2021).
Ji, W. et al. Rigid tightly packed amino acid crystals as functional supramolecular materials. ACS Nano 13, 14477–14485 (2019).
Zhdanova, N. G. et al. Tyrosine fluorescence probing of the surfactant-induced conformational changes of albumin. Photochem. Photobiol. Sci. 14, 897–908 (2015).
Jang, H.-S. et al. Tyrosine-mediated two-dimensional peptide assembly and its role as a bio-inspired catalytic scaffold. Nat. Commun. 5, 3665 (2014).
Brillante, B. A. et al. Characterization of phase purity in organic semiconductors by lattice-phonon confocal Raman mapping: application to pentacene. Adv. Mater. 17, 2549–2553 (2005).
Xing, R., Yuan, C., Fan, W., Ren, X. & Yan, X. Biomolecular glass with amino acid and peptide nanoarchitectonics. Sci. Adv. 9, eadd8105 (2023).
Yokota, H., Sakata, H., Nishibori, M. & Kinosita, K. Ellipsometric study of polished glass surfaces. Surf. Sci. 16, 265–274 (1969).
Jeener, J., Meier, B. H., Bachmann, P. & Ernst, R. R. Investigation of exchange processes by two‐dimensional NMR spectroscopy. J. Chem. Phys. 71, 4546–4553 (1979).
Hibbert, F. & Emsley, J. Hydrogen bonding and chemical reactivity. Adv. Phys. Org. Chem. 26, 255–379 (1990).
Davis, J. H., Jeffrey, K. R., Bloom, M., Valic, M. I. & Higgs, T. P. Quadrupolar echo deuteron magnetic resonance spectroscopy in ordered hydrocarbon chains. Chem. Phys. Lett. 42, 390–394 (1976).
Larsen, F. H. in Annual Reports on NMR Spectroscopy Vol. 71 (ed. Webb, G. A.) 103–137 (Elsevier, 2010).
Hernández, B., Coïc, Y., Pflüger, F., Kruglik, G. & Ghomi, M. All characteristic Raman markers of tyrosine and tyrosinate originate from phenol ring fundamental vibrations. J. Raman Spectrosc. 47, 210–220 (2016).
Ihli, J. et al. Mechanical adaptation of brachiopod shells via hydration-induced structural changes. Nat. Commun. 12, 5383 (2021).
Ge, H. et al. Fracture behavior of colloidal polymer particles in fast-frozen suspensions viewed by cryo-SEM. Macromolecules 39, 5531–5539 (2006).
Desloir, M., Benoit, C., Bendaoud, A., Alcouffe, P. & Carrot, C. Plasticization of poly(vinyl butyral) by water: glass transition temperature and mechanical properties. J. Appl. Polym. Sci. 136, 47230 (2019).
Kilburn, D. et al. Water in glassy carbohydrates: opening it up at the nanolevel. Phys. Chem. 33, 12436–12441 (2004).
Flores, A., Ania, F. & Baltá-Calleja, F. J. From the glassy state to ordered polymer structures: A microhardness study. Polymer 50, 729–746 (2009).
Wang, Q., Chen, H., Wang, Y. & Sun, J. Thermal shock effect on the glass thermal stress response and crack propagation. Procedia Eng. 62, 717–724 (2013).
Frankberg, E. J. et al. Highly ductile amorphous oxide at room temperature and high strain rate. Science 366, 864–869 (2019).
Delaglio, F. et al. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Lee, W., Tonelli, M. & Markley, J. L. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics 31, 1325–1327 (2015).
Acknowledgements
This work was supported by the Air Force Office of Scientific Research under award no. FA8655-21-1-7004 (E.G.), the Marian Gertner Institute for Medical Nanosystems Research (G.F.-Z. and Z.A.A.), the Clore Scholars Program, the Zuckerman Israeli-Postdoc Program and the Human Frontier Science Program (HFSP LT000158/2021-C) (Z.A.A.). T.V. thanks Tel Aviv University for the post-doctoral fellowship. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the US Air Force. We thank T. Brosh and L. Adler-Abramovich for assisting with the mechanical measurements, B. Ratzker for helping in the design of the measurements, D. Rachmiel for the assistance with drilling holes in the glass slides and D. Zaguri for providing us with the weights. We also thank R. Beck and G. R. Koren for their assistance in SAXS measurements. We thank S. S. B. Shabtay and Y. Cohen for their assistance in the NMR measurements, J. Jopp for the infrared measurements and D. Levy for the PXRD measurements. Last, we thank all members of the Gazit group for their helpful discussions.
Author information
Authors and Affiliations
Contributions
G.F.-Z., Z.A.A. and E.G. conceptualized the project. G.F.-Z., Z.A.A., T.V. and E.G. conceived and designed the methodology. G.F.-Z., M.S. and O.M. performed the characterization of the mechanical properties. A.G., O.S.L. and T.V. performed the NMR experiments and analysed them. B.A.P. and A.W. performed cryo-SEM measurements. G.F.-Z., G.Z. and R.A. performed the transparency measurements. E.G., T.E., Z.A.A. and L.M. designed and performed the optical lens experiment. S.R.-L. and S.G. contributed to the interpretation of the data and text editing. S.S. synthesized and characterized the peptide derivative. M.J.P. performed the Raman experiment. D.A.G. and S.K. assisted with the sample preparation. T.E., B.A.P., A.G., M.S. and E.G. provided resources for this project. E.G. supervised the work and performed project administration. G.F.-Z., Z.A.A. and E.G. wrote the Article. All authors discussed the results, provided intellectual input and critical feedback and commented on the Article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Olivier Lebel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–17, Supplementary Table 1 and Supplementary Schemes 1 and 2.
Supplementary Video 1
Successful adhesion of two microscope glass slides held together by a YYY adhesive layer, under a downward load of 5 kg.
Supplementary Video 2
Cracks propagation in a YYY peptide glass under dry conditions.
Supplementary Video 3
Self-healing of a cracked YYY peptide glass under humid conditions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Finkelstein-Zuta, G., Arnon, Z.A., Vijayakanth, T. et al. A self-healing multispectral transparent adhesive peptide glass. Nature 630, 368–374 (2024). https://doi.org/10.1038/s41586-024-07408-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07408-x
This article is cited by
-
Context dependence in assembly code for supramolecular peptide materials and systems
Nature Reviews Materials (2025)
-
Design and sustainability of polypeptide material systems
Nature Reviews Materials (2025)
-
Self-healing crystals
Nature Reviews Chemistry (2025)
-
Adaptive peptide dispersions enable drying-induced biomolecule encapsulation
Nature Materials (2025)
-
Supramolecular glass: a new platform for ultralong phosphorescence
Science China Materials (2024)