Abstract
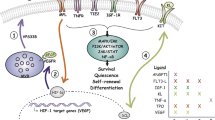
Transition between activation and quiescence states in hematopoietic stem and progenitor cells (HSPCs) is tightly governed by cell-intrinsic means and microenvironmental co-adaptation. Although this balance is fundamental for lifelong hematopoiesis and immunity, the underlying molecular mechanisms remain poorly defined. Multimodal analysis divulging differential transcriptional activity between distinct HSPC states indicates the presence of Fli-1 transcription factor binding motif in activated hematopoietic stem cells. We reveal that Fli-1 activity is essential during regenerative hematopoiesis in mice. Fli-1 directs activation programs while priming cellular sensory and output machineries, enabling HSPCs co-adoptability with a stimulated vascular niche through propagation of niche-derived angiocrine Notch1 signaling. Constitutively induced Notch1 signaling is sufficient to recuperate functional hematopoietic stem cells impairments in the absence of Fli-1, without leukemic transformation. Applying FLI-1 transient modified-mRNA transduction into latent adult human mobilized HSPCs, enables their niche-mediated expansion and superior engraftment capacities. Thus, decryption of stem cell activation programs offers valuable insights for immunological regenerative medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Omics data that were generated for or retrieved for this manuscript have been deposited in the Gene Expression Omnibus or in the European Genome-Phenome repositories under the following accession codes:
Multiomics SuperSeries, GSE169431; mouse HSPC bulk Fli-1 and Spi1 ChIP–seq, GSE22178; human HSPC bulk FLI-1 ChIP–seq, GSE45144; human HSPC bulk RNA-seq, GSE125345; human HSPC scATAC-seq, EGAS00001004740; and human HSPC bulk ATAC-seq, EGAS00001004742. Human targeted DNA-seq data are available on Zenodo at https://doi.org/10.5281/zenodo.10049312 (ref. 67). Source data are provided with this paper.
Code availability
New customized codes were not developed in this study.
References
Yu, V. W. C. et al. Epigenetic memory underlies cell-autonomous heterogeneous behavior of hematopoietic stem cells. Cell 168, 944–945 (2017).
Wu, Q. et al. Resilient anatomy and local plasticity of naive and stress haematopoiesis. Nature 627, 839–846 (2024).
Itkin, T. et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328 (2016).
Baccin, C. et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 22, 38–48 (2020).
Man, Y., Yao, X., Yang, T. & Wang, Y. Hematopoietic stem cell niche during homeostasis, malignancy, and bone marrow transplantation. Front. Cell Dev. Biol. 9, 621214 (2021).
Zhou, B. O., Ding, L. & Morrison, S. J. Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1. eLife 4, e05521 (2015).
Baryawno, N., Severe, N. & Scadden, D. T. Hematopoiesis: reconciling historic controversies about the niche. Cell Stem Cell 20, 590–592 (2017).
Rodriguez-Fraticelli, A. E. et al. Single-cell lineage tracing unveils a role for TCF15 in haematopoiesis. Nature 583, 585–589 (2020).
Chavez, J. S. et al. PU.1 enforces quiescence and limits hematopoietic stem cell expansion during inflammatory stress. J. Exp. Med. 218, e20201169 (2021).
Oh, I. H. & Eaves, C. J. Overexpression of a dominant negative form of STAT3 selectively impairs hematopoietic stem cell activity. Oncogene 21, 4778–4787 (2002).
Kato, Y. et al. Selective activation of STAT5 unveils its role in stem cell self-renewal in normal and leukemic hematopoiesis. J. Exp. Med. 202, 169–179 (2005).
Kim, T. G. et al. CCCTC-binding factor is essential to the maintenance and quiescence of hematopoietic stem cells in mice. Exp. Mol. Med. 49, e371 (2017).
Komorowska, K. et al. Hepatic leukemia factor maintains quiescence of hematopoietic stem cells and protects the stem cell pool during regeneration. Cell Rep. 21, 3514–3523 (2017).
Gomez-Salinero, J. M. et al. Cooperative ETS transcription factors enforce adult endothelial cell fate and cardiovascular homeostasis. Nat. Cardiovasc. Res. 1, 882–899 (2022).
Loughran, S. J. et al. The transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat. Immunol. 9, 810–819 (2008).
Li, Y., Luo, H., Liu, T., Zacksenhaus, E. & Ben-David, Y. The ets transcription factor Fli-1 in development, cancer and disease. Oncogene 34, 2022–2031 (2015).
Spyropoulos, D. D. et al. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol. Cell. Biol. 20, 5643–5652 (2000).
Starck, J. et al. Inducible Fli-1 gene deletion in adult mice modifies several myeloid lineage commitment decisions and accelerates proliferation arrest and terminal erythrocytic differentiation. Blood 116, 4795–4805 (2010).
Palii, C. G. et al. Single-cell proteomics reveal that quantitative changes in co-expressed lineage-specific transcription factors determine cell fate. Cell Stem Cell 24, 812–820 (2019).
Chen, Z. et al. In vivo CD8+ T cell CRISPR screening reveals control by Fli1 in infection and cancer. Cell 184, 1262–1280 (2021).
Butler, J. M. et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251–264 (2010).
Le Bouteiller, M. et al. Notchless-dependent ribosome synthesis is required for the maintenance of adult hematopoietic stem cells. J. Exp. Med. 210, 2351–2369 (2013).
Yang, Y. et al. The histone lysine acetyltransferase HBO1 (KAT7) regulates hematopoietic stem cell quiescence and self-renewal. Blood 139, 845–858 (2022).
Arif, T. et al. Activated but not quiescent hematopoietic stem cells rely readily on glycolysis as their main source of energy. Blood 134, 271–271 (2019).
Staber, P. B. et al. Sustained PU.1 levels balance cell-cycle regulators to prevent exhaustion of adult hematopoietic stem cells. Mol. Cell 49, 934–946 (2013).
Sato, T. et al. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat. Med. 15, 696–700 (2009).
Wilson, N. K. et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell 7, 532–544 (2010).
Kaur, S. et al. Stable colony-stimulating factor 1 fusion protein treatment increases hematopoietic stem cell pool and enhances their mobilisation in mice. J. Hematol. Oncol. 14, 3 (2021).
Pruijt, J. F., Lindley, I. J., Heemskerk, D. P., Willemze, R. & Fibbe, W. E. Leukemia inhibitory factor induces in vivo expansion of bone marrow progenitor cells that accelerate hematopoietic reconstitution but do not enhance radioprotection in lethally irradiated mice. Stem Cells 15, 50–55 (1997).
Weksberg, D. C., Chambers, S. M., Boles, N. C. & Goodell, M. A. CD150- side population cells represent a functionally distinct population of long-term hematopoietic stem cells. Blood 111, 2444–2451 (2008).
Gur-Cohen, S. et al. PAR1 signaling regulates the retention and recruitment of EPCR-expressing bone marrow hematopoietic stem cells. Nat. Med. 21, 1307–1317 (2015).
Tikhonova, A. N. et al. The bone marrow microenvironment at single-cell resolution. Nature 569, 222–228 (2019).
Varnum-Finney, B. et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat. Med. 6, 1278–1281 (2000).
Stier, S., Cheng, T., Dombkowski, D., Carlesso, N. & Scadden, D. T. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood 99, 2369–2378 (2002).
Duncan, A. W. et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 6, 314–322 (2005).
Scheicher, R. et al. CDK6 as a key regulator of hematopoietic and leukemic stem cell activation. Blood 125, 90–101 (2015).
Garcia-Prat, L. et al. TFEB-mediated endolysosomal activity controls human hematopoietic stem cell fate. Cell Stem Cell 28, 1838–1850 (2021).
Buonamici, S. et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 459, 1000–1004 (2009).
Chiang, M. Y., Shestova, O., Xu, L., Aster, J. C. & Pear, W. S. Divergent effects of supraphysiologic Notch signals on leukemia stem cells and hematopoietic stem cells. Blood 121, 905–917 (2013).
Wilson, N. K. et al. Combined single-cell functional and gene expression analysis resolves heterogeneity within stem cell populations. Cell Stem Cell 16, 712–724 (2015).
Chen, J., Lazarus, H. M., Dahi, P. B., Avecilla, S. & Giralt, S. A. Getting blood out of a stone: identification and management of patients with poor hematopoietic cell mobilization. Blood Rev. 47, 100771 (2021).
Butler, J. M. et al. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood 120, 1344–1347 (2012).
Boitano, A. E. et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329, 1345–1348 (2010).
Wilkinson, A. C. et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 571, 117–121 (2019).
Venezia, T. A. et al. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2, e301 (2004).
Roy, A. et al. Transitions in lineage specification and gene regulatory networks in hematopoietic stem/progenitor cells over human development. Cell Rep. 36, 109698 (2021).
Takayama, N. et al. The transition from quiescent to activated states in human hematopoietic stem cells is governed by dynamic 3D genome reorganization. Cell Stem Cell 28, 488–501 (2021).
Beck, D. et al. Genome-wide analysis of transcriptional regulators in human HSPCs reveals a densely interconnected network of coding and noncoding genes. Blood 122, e12–e22 (2013).
Chien, K. R., Zangi, L. & Lui, K. O. Synthetic chemically modified mRNA (modRNA): toward a new technology platform for cardiovascular biology and medicine. Cold Spring Harb. Perspect. Med. 5, a014035 (2014).
Magadum, A. et al. Pkm2 regulates cardiomyocyte cell cycle and promotes cardiac regeneration. Circulation 141, 1249–1265 (2020).
Kaufmann, K. B. et al. A latent subset of human hematopoietic stem cells resists regenerative stress to preserve stemness. Nat. Immunol. 22, 723–734 (2021).
Cheng, D. T. et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 17, 251–264 (2015).
Kornblau, S. M. et al. Abnormal expression of FLI1 protein is an adverse prognostic factor in acute myeloid leukemia. Blood 118, 5604–5612 (2011).
Naldini, L. Genetic engineering of hematopoiesis: current stage of clinical translation and future perspectives. EMBO Mol. Med. 11, e9958 (2019).
Ferrari, S. et al. Efficient gene editing of human long-term hematopoietic stem cells validated by clonal tracking. Nat. Biotechnol. 38, 1298–1308 (2020).
Gothert, J. R. et al. In vivo fate-tracing studies using the Scl stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood 105, 2724–2732 (2005).
Barcia Duran, J. G., Lis, R., Lu, T. M. & Rafii, S. In vitro conversion of adult murine endothelial cells to hematopoietic stem cells. Nat. Protoc. 13, 2758–2780 (2018).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Stuart, T., Srivastava, A., Madad, S., Lareau, C. A. & Satija, R. Single-cell chromatin state analysis with Signac. Nat. Methods 18, 1333–1341 (2021).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Haghverdi, L., Lun, A. T. L., Morgan, M. D. & Marioni, J. C. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat. Biotechnol. 36, 421–427 (2018).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. https://doi.org/10.1038/nbt.4314 (2019).
Pliner, H. A., Shendure, J. & Trapnell, C. Supervised classification enables rapid annotation of cell atlases. Nat. Methods 16, 983–986 (2019).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Sriramkumar, S. et al. Single-cell analysis of a high-grade serous ovarian cancer cell line reveals transcriptomic changes and cell subpopulations sensitive to epigenetic combination treatment. PLoS ONE 17, e0271584 (2022).
Houghton, S. Targeted DNA-seq analysis was performed on sorted population of CD45+/CD34+ HSPCs from control or FLI-1 modified mRNA treated mPB after co-culture with vascular niche cells. Zenodo https://doi.org/10.5281/zenodo.10049312 (2023).
Acknowledgements
This study was primarily supported by the Hartman Institute for Therapeutic Organ Regeneration, Ansary Stem Cell Institute and National Institutes of Health (NIH) grants R35 HL150809, R01DK136327, U01AI138329 and the Selma and Lawrence Ruben Daedalus Fund for Innovation (to T.I., S.R., S.H., F.G., R.S., Y.L., D.R. and J.M.G.S.). We are grateful to the American Society of Hematology (ASH)-European Hematology Association (EHA) Translational Research Training in Hematology (TRTH) joint program for the valuable support and guidance of T.I. and this study. Y. Liu and J.M.G.-S. are New York Stem Cell Foundation-Druckenmiller fellows. Y. Lin is a NYSTEM fellow. L.G.-P. is supported by an EMBO long-term fellowship, a Benjamin Pearl fellowship and a CIHR fellowship. We thank members of Weill Cornell Medicine’s Genomic Core facility for all their tremendous help and assistance. We acknowledge the use of the Integrated Genomics Operation Core, funded by the NCI Cancer Center Support Grant (CCSG, P30 CA08748), Cycle for Survival, and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology. This study was supported and partially funded by the following grants and agencies: Tel Aviv University’s Sagol Center for Regenerative Medicine and Sheba Medical Center’s Research Authority Startup Funds (to T.I.), NYSTEM grant NYSTEM-C32596GG (to R.S.), NIH grants R01HL168110, RC2DK114777 and K08HL140143 (to B.H.), Princess Margaret Cancer Centre Foundation (to J.E.D. and S.Z.X.), Canadian Institutes of Health Research (foundation no. 154293, operating grant nos. 154293 and 89932), International Development Research Centre, Canadian Cancer Society (grant no. 703212), Terry Fox Research Institute Program Project grant, and Canada Research Chair and the Ontario Ministry of Health and Long Term Care (to J.E.D.), UC Merced Startup Funds and NSF-CREST NSF-HRD-1547848 and NSF-HRD-2112675 funds (to J.A.S.), NCI award K08CA267058 (to W.X.), NCI award R35CA197594 (to R.L.), MSKCC Support Grant/Core Grant P30 CA008748 and Tri-Institutional Stem Cell Initiative that is supported by The Starr Foundation (to W.X. and R.L.) and Leukemia and Lymphoma Society Scholar Award and NIH R01 DK119394 (to E.M.P.). Illustrations in this manuscript were partially or entirely created with BioRender.com.
Author information
Authors and Affiliations
Contributions
T.I., S.H. and S.R. conceived and designed the study, supervised experiments and analysis, performed experiments, analyzed data and wrote the manuscript. Y. Lin, C.R.B., Y. Liu, J.M.G.-S., F.G. and J.-H.S. performed experiments and assisted in data analysis. B.H. designed and together with T.I. analyzed scRNA-seq data. J.E.D., S.Z.X., V.V., A.M., K.B.K. and L.G.-P. designed, performed and analyzed bulk RNA-seq experiments of human CB and mPB HSPCs. J.A.S. designed and together with N.S. performed intravital imaging experiments and analyzed data. S.H. designed and wrote analysis scripts, and with T.I. performed bioinformatic analyses of bulk RNA-seq, ATAC-seq, multiple types of ChIP–seq, scRNA-seq and single-nuclei multiome data. S.Z.J. assisted in design and analysis of ATAC-seq-related experiments. D.R. participated in some computational RNA-seq analyses. C.T., G.B., S.H. and T.I. designed, performed and analyzed multiome snRNA/ATAC-seq analysis. R.S. designed and performed microscopy experiments and analyzed imaging data. J.Z.X. assisted with design and performance of all sequencing analysis. L.Z. designed and synthesized modified mRNA and assisted in design of modified-mRNA-related experiments. E.M.P. designed Spi1 ChIP–seq analysis and provided experimental raw data. R.L. and W.X. designed, performed and analyzed targeted DNA-sequencing analysis.
Corresponding authors
Ethics declarations
Competing interests
S.R. is the founder and an unpaid consultant to Angiocrine Bioscience. J.E.D. declares research funding from BMS and licensing of SIRP-alpha to Trillium Therapeutics and Pfizer. All other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Joao Pereira, Toshio Suda, Bradley Blaser, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
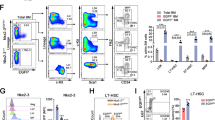
Extended Data Fig. 1 Fluorescence-activated cell sorting gating strategies for flow cytometry analyses.
a, Panels I-VI represent the gating strategy for flow cytometry analysis and sorting of single cell Live Lineage-Sca-1+c-Kit+ HSPC and Panels I-VII for single cell Live Lineage-Sca-1+c-Kit+CD150+CD48- HSPC. This gating strategy was applied in Fig.1, Extended Data Figs. 2–4, and 7. b, Following the same gating strategy of panels (a)I-IV, panels V, VI, and VII were used to determine CD45.2+ cells in chimeric mice and their T cell, B cell, and myeloid cell. This gating strategy was applied in Fig. 4 and Extended Data Figs. 2,3,7, and 9. c, Following the same gating strategy of panels (a)I-IV, panel V presents the gating strategy for in vitro co-cultured murine HSPC as Lineage-human CD31- excluding human vascular endothelial niche cells. For Lineage-Sca-1+c-Kit+ HSPC we further gated for the upper right quadrant as it appears in Extended Data Fig. 7f. This gating strategy was applied in Figs. 2–4 and Extended Data Figs. 4–8. d, Following the same gating strategy of panels (a)I-IV, panel V presents the gating strategy for co-cultured and transplanted human hematopoietic cells as human CD45+human CD31- excluding human vascular endothelial niche cells. For CD45+CD34+ human HSPC and for CD45+CD34+CD38- human HSPC we further gated as it appears in Fig. 7d. This gating strategy was applied in Figs. 6–8 and Extended Data Fig. 10.
Extended Data Fig. 2 Hematopoietic Fli-1 deficiency leads to thrombocytopenia and to HSC regenerative failure.
a, Survival plot for WT, Fli-1ROSAΔ, and WT/Fli-1ROSAΔ (heterozygous) mice, following the beginning of tamoxifen induction (1st injection on day 0). Gehan-Breslow-Wilcoxon test was used; n = 9 (WT), n = 11 (Fli-1ROSAΔ), and n = 13 (WT/Fli-1ROSAΔ). b, Illustration of the working model to generate hematopoietic specific and inducible Fli-1ROSAΔ mice. WT mice are lethally irradiated (475 rad + 475 rad with 5 hours interval) and are transplanted with 2×105 Lineage depleted (Linneg) BM cells from either WT or Fli-1ROSAΔ mice. Four months (16 weeks) post-transplant (full hematopoietic recovery) mice are administrated with tamoxifen to induce Fli-1 gene deletion. c, Survival plot for WT mice with either WT, Fli-1ROSAΔ, and WT/Fli-1ROSAΔ (heterozygous) hematopoietic cells, following the beginning of tamoxifen induction (1st injection on day 0). Gehan-Breslow-Wilcoxon test was used; n = 9 (WT), n = 10 (Fli-1ROSAΔ), and n = 11 (WT/Fli-1ROSAΔ). d, Representative image of ear cuts from WT (upper) and Fli-1ROSAΔ (lower) mice. e, A representative H&E image of femoral BM section from WT and Fli-1ROSAΔ mice at day 7 post induction (n = 5 mice per genotype). Bar = 50 µM. Yellow arrows indicate WT and abnormal Fli-1ROSAΔ megakaryocytes. f-j, At day 7 post induction blood was collected and hematological parameters were scored using ADVIA 120. Platelet counts for WT and Fli-1ROSAΔ mice are presented per 1 µL of blood. White blood cell (WBC) and red blood cell (RBC) counts are presented per 1 µL of blood. Hemoglobin (HGB) is presented in grams per deciliter. Hematocrit (HCT) frequency in blood is presented. Unpaired two tailed t-test was used; n = 5 per WT or Fli-1ROSAΔ. Data are presented as mean values + SEM. k, At day 7 post induction femurs were flushed, stained with Turk’s solution and WBC were counted and determined using hemacytometer. Unpaired two tailed t-test was used; n = 7 mice per genotype. Data are presented as mean values + SEM. l, Number of scored BM derived colonies per sub-type for WT and Fli-1ROSAΔ mice at day 7 post induction. Multiple unpaired t-tests were performed; n = 7 per WT or Fli-1ROSAΔ with each n representing an average of two replicates. Data are presented as mean values + SEM. m, n, Number of BM LSK HSPC and SLAM LSK HSPC as determined by flow cytometry and normalized per WBC counts per femur at day 7 post induction. Unpaired two tailed t-test was used; n = 7 per WT or Fli-1ROSAΔ. Data are presented as mean values +/- SEM. o, Illustration of the working flow to generate hematopoietic specific and inducible Fli-1ROSAΔ mice, followed by Fli-1 knockout induction, and 2ndery transplant. WT mice are lethally irradiated (475 rad + 475 rad with 5 hours interval) and are transplanted with 2×105 Lineage depleted (Linneg) BM cells from either WT or Fli-1ROSAΔ mice. Four months (16 weeks) post-transplant (full hematopoietic recovery) mice are administrated with tamoxifen to induce Fli-1 gene deletion. Seven days post induction bones were collected, LSK HSPCs were sorted and competitively co-transplanted with congenic SJL BM cells into lethally irradiated congenic SJL mice. p, On day 7 post induction WT or Fli-1ROSAΔ BM LSK HSPC were isolated by flow cytometry sorting (n = 8 per genotype) and co-transplanted competitively with congenic SJL total BM cells into lethally irradiated congenic SJL recipient mice. Every 4 weeks peripheral blood was collected and chimerism frequency was determined by flow cytometry. Multiple unpaired t-tests were performed. ****p < 0.0001. Data are presented as mean values +/- SEM.
Extended Data Fig. 3 Fli-1 deficient HSPC regenerative failure is due to hampered hematopoiesis rather than homing defects.
BM WT and Fli-1ROSAΔ Lineage depleted cells were collected and plated on top of a vascular niche layer. After 48 hours 4-hydroxytamoxifen (4-OHT) was administrated to induce Fli-1 deletion. After additional 48 hours LSK HSPCs were sorted and frozen for further experiments. Thawed WT and Fli-1ROSAΔ HSPCs were labeled with either DiI or DiD dyes (10 µM) (swapping dye labeling per genotype between experiments was performed to ensure no dye related effects). Recipient mice were sub-lethally irradiated (300 rad + 300 rad with 5 hours interval) 24 hours before transplantation. WT and Fli-1ROSAΔ HSPCs were co-injected into recipient mice and 24 hours later mouse calvarias were imaged and cell luminal or abluminal location was determined using multiphoton/confocal laser scanning video-rate microscopy. a, Upper representative image – DiD labeled Fli-1ROSAΔ HSPC (red arrow) and autofluorescence (red). DiI labeled WT HSPC (white arrow), autofluorescence, and dextran labeled blood vessels (blue). Bone - second harmonic generation of collagen (green). Lower representative image – DiI labeled Fli-1ROSAΔ HSPCs (yellow arrows), autofluorescence, and dextran labeled blood vessels (blue). Bone - second harmonic generation of collagen (green). Note vessel wedged Fli-1ROSAΔ HSPCs at lower panel image. Bars = 50 µM. See also Movies S1 and S2. b, Bars and pies presenting the portion of HSPCs located in abluminal/intramarrow (homed) or luminal/extramarrow (not homed) locations. Unpaired two tailed t-test was used; n = 3 biological repeats of experiment per genotype with n = 55 single HSPC events imaged and recorded. Data are presented as mean values + SEM. c-j, Lin- WT or Fli-1ROSAΔ BM cells mixed with congenic Lin- SJL BM cells (1:1 ratio) were co-transplanted into congenic SJL lethally irradiated recipient mice. Data are presented as mean values +/- SEM. c, Illustration of the working flow to generate mice with mixed chimeric hematopoietic system containing either C57BL/6 and SJL WT or a mix of SJL WT and C57BL/6 inducible Fli-1ROSAΔ hematopoietic cells, to allow the study of Fli-1 deficient HSCs in a microenvironment containing WT hematopoietic and stromal cells. Mixed cells were co-transplanted into congenic SJL lethally irradiated recipient mice. Seven days post-transplant (to ensure successful hematopoietic BM lodgment), and four months post-transplant (to ensure complete hematopoietic BM recovery), tamoxifen was administrated for induction of Fli-1 deletion. d, Seven days post-transplant, to ensure successful hematopoietic BM lodgment, tamoxifen was administrated for induction. Every 4 weeks post induction peripheral blood was collected and levels of chimerism were determined by flow cytometry. Multiple unpaired t-tests were performed; n = 15 per WT and n = 10 per Fli-1ROSAΔ. ****p < 0.0001. e-h, Four months post-transplant, to ensure complete hematopoietic BM recovery, tamoxifen was administrated for induction. On day 0 Prior to induction and at indicated time points post induction peripheral blood was collected and levels of chimerism were determined by flow cytometry among total hematopoietic cells, and myeloid/lymphoid lineage specific cells. Multiple unpaired t-tests were performed; n = 8 per genotype. ***p < 0.001, ****p < 0.0001. i-j, Eight weeks post induction, BM cells were collected and apoptosis (i) or quiescence (j) frequency among WT or Fli-1ROSAΔ LSK and SLAM LSK HSPCs was determined by flow cytometry. Unpaired two tailed t-test was used; n = 6 per genotype (i) and n = 5 per genotype (j).
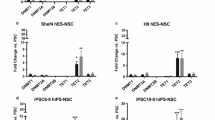
Extended Data Fig. 4 HSPC specific deletion of Fli-1 hampers regenerative activation programs in vivo and in vitro.
a-h, “HSC specific” Scl-CreERT2 with ROSA-Tomato reporter mice (WT) and Scl-CreERT2 with ROSA-Tomato and with Fli-1flx/flx mice (Fli-1SCLΔ) were treated for 5 days with 4-OHT. On day 7 mouse BM cells were analyzed by flow cytometry and either WT or Fli-1SCLΔ Tomato+ LSK cells were sorted and co-transplanted (in 1:1 ratio) with WT C57Bl/6 sorted LSK cells into lethally irradiated C57Bl/6 mice. Data are presented as mean values +/- SEM. a, b, Number of BM LSK HSPC and SLAM LSK HSPC as determined by flow cytometry and normalized per WBC counts per femur. Unpaired two tailed t-test was used; n = 5 per WT or Fli-1SCLΔ. c,d, Frequency of Tomato(TMT)+ BM LSK HSPC and SLAM LSK HSPC as determined by flow cytometry. Unpaired two tailed t-test was used; n = 5 per WT or Fli-1SCLΔ. e, Representative histogram plot of Tomato expression in WT (top) or Fli-1SCLΔ (bottom) SLAM LSK HSPC. Histogram plot bars indicate the gates for the positive fluorescent signal. f, Frequency of PB circulating Tomato+CD45+ cells as determined by flow cytometry 4 weeks post transplantation. Unpaired two tailed t-test was used; n = 5 per WT or Fli-1SCLΔ with each original donor mouse transplanted into n = 3 recipients. g, Representative flow cytometry density plots of PB circulating Tomato+CD45+ cells in WT (upper panel) and Fli-1SCLΔ (lower panel) mice. h, Frequency of BM engrafted Tomato+CD45+ cells as determined by flow cytometry 4 weeks post transplantation. Unpaired two tailed t-test was used; n = 5 per WT or Fli-1SCLΔ with each original donor mouse transplanted into n = 3 recipients. i, Illustrated working flow for studies of adult HSPC expansion in co-culture with a vascular niche. Bones are collected from adult WT or Fli-1ROSAΔ mice and LSK HSPCs were isolated. Next, HSPCs were co-cultured with a layer of a vascular niche for 48 h to allow recovery and induction of cellular expansion program. After 48 h 4-OHT is added to the co-cultures to induce Fli-1 deletion, and co-cultures are kept for additional 6 days, supplemented with cytokine containing serum free media every other day. j, Frequency of LSK HSPCs was determined by flow cytometry and fold expansion was calculated. One-way ANOVA multiple comparisons was used; n = 6 BM donor mice per genotype, each point represents an average of 3 technical replicates. Data are presented as mean values +/- SEM. k, Transcription factor binding site motif enrichment analysis for downregulated ATAC- and H3K27ac ChIP-seq peak sites in Fli-1ROSAΔ vs. WT HSPCs. See also Supplementary Data 2,3. Created with BioRender.com.
Extended Data Fig. 5 Fli-1 deficient HSPCs exhibit defusing of activation programs and parallel induction of Spi1-mediated quiescence programs.
a, BETA motif analysis on Fli-1 target genes downregulated in Fli-1ROSAΔ HSPCs, showing the top scored motifs (upper panel) and the top scored motif for Fli-1 target genes vs. non-targeted genes downregulated in Fli-1ROSAΔ HSPCs (lower panel). b, BETA activating/repressive function prediction analysis of the Spi1 binding data sets from in vivo sorted dormant HSPCs and expression data sets from Fli-1ROSAΔ vs. WT HSPCs, identifying upregulated (red) and downregulated (purple) genes. The dashed line indicates the non-differentially expressed genes as background. c, d, GSEA analysis plot for HSC quiescence (c) and HSC activation (d) factors expression in Fli-1ROSAΔ vs. WT HSPCs, showing a positive enrichment for quiescence factors and negative enrichment for activation factors in Fli-1ROSAΔ HSPCs. e, Heatmap for selected HSC quiescence (upper panel) and HSC activation (lower panel) factors across RNA-seq replicates of Fli-1ROSAΔ and WT HSPCs. Scale bar and coloring represent the Z-score scaled by gene for Log counts per 106 normalized by library size (trimmed mean of M-values). f, Bars displaying the number of predicted active enhancers, following activity-by-contact (ABC) model analysis, per each quiescence factor for WT (lower panel) and Fli-1ROSAΔ (upper panel) HSPCs. Also, presenting a heatmap for the averaged ABC PowerLaw score for the predicted enhancers per quiescence factor in WT and Fli-1ROSAΔ HSPCs. g, Frequency of cycling HSPCs in co-culture as determined by flow cytometry on day 4 post 4-OHT induction. Unpaired two tailed t-test was used; n = 5 per WT or Fli-1SCLΔ. Data are presented as mean values +/- SEM. h, IGV peaks plots for the genomic loci of Spi1 (left) and Fli-1 (right), displaying bigWig coverage tracks for WT and Fli-1ROSAΔ ATAC-seq, H3K27ac-ChIP-seq, Fli1-ChIP-seq, and Spi1-ChIP-seq.
Extended Data Fig. 6 Intersecting transcriptional and chromatin accessibility data reveals HSPC sensory and cytokine production deficiencies in the absence of Fli-1.
a, Intersection dot plot of RNA-seq and ATAC-seq in Fli-1ROSAΔ vs. WT HSPCs. Each dot represents the transcriptional and chromatic accessibility statuses per gene. For gene labeling of few selected dots, thresholds FDR < 0.05 for both RNA- and ATAC-seq, |LogFC | >2.5 for RNA-seq, |LogFC | >0.75 for ATAC-seq, and |Distance to TSS | < 500 parameters were set. b, Gene ontology (GO) categories of biological processes enrichment for significantly downregulated genes in both RNA- and ATAC-seq analyses of Fli-1ROSAΔ vs. WT HSPCs (lower left quadrant in panel a). Significance by FDR value is indicated. c, d, Heatmaps for selected HSC extra-sensory quiescence (c) and activation (d) elements across RNA-seq replicates of Fli-1ROSAΔ and WT HSPCs. Scale bar and coloring represent the Z-score scaled by gene for Log counts per 106 normalized by library size (trimmed mean of M-values). e, Frequency of double positive HSPCs for the activation receptors Lifr and Csfr1 in co-culture as determined by flow cytometry on day 4 post 4-OHT induction. Unpaired two tailed t-test was used; n = 5 per WT or Fli-1ROSAΔ. Data are presented as mean values +/- SEM. f, Representative flow cytometry dot plots of Lifr+/Csfr1+ double positive HSPCs in WT (upper panel) and Fli-1ROSAΔ (lower panel) co-cultures. g, h, Mean fluorescent intensity expression of stem markers CD150 (g) and EPCR (h) on LSK HSPCs in co-culture as determined by flow cytometry on day 4 post 4-OHT induction. Unpaired two tailed t-test was used; n = 5 per WT or Fli-1ROSAΔ. Data are presented as mean values +/- SEM.
Extended Data Fig. 7 Restoring Notch1 signaling in Fli-1 deficient HSPCs rescues their expansion capacities.
a, Frequency of Notch1+ BM LSK and SLAM LSK HSPC from WT and Fli-1ROSAΔ chimeric mice (described in Extended Data Figs. 2b), 7 days post tamoxifen induction. Unpaired two tailed t-test was used; n = 5 per WT or Fli-1ROSAΔ. Data are presented as mean values +/- SEM. b, c, WT and Fli-1ROSAΔ BM HSPCs were isolated and expanded. b, Flow cytometry determined frequency of Vegfa expressing LSK HSPCs. Unpaired two tailed t-test was used; n = 5 tissue donor mice per genotype, each point represents an average of 3-4 technical replicates. Data are presented as mean values +/- SEM. c, Representative Vegfa histogram plots gated from LSK HSPCs. Histogram plot bars indicate the gates for the positive fluorescent signal. d, Frequency of Vegfa+ BM LSK and SLAM LSK HSPC from WT and Fli-1ROSAΔ chimeric mice (described in Extended Data Figs. 1b), 7 days post tamoxifen induction. Unpaired two tailed t-test was used; n = 5 per WT or Fli-1ROSAΔ. Data are presented as mean values +/- SEM. e, IGV peaks plot for the genomic loci of Vegfa, displaying bigWig coverage tracks for Fli1-ChIP-seq and Spi1-ChIP-seq. f, Representative flow dot plots for WT, Fli-1ROSAΔ, Fli-1ROSAΔN1-ICiOE, and N1-ICiOE HSPCs post expansion in co-culture with a vascular niche, gated from CD45+/Lin- cells. g, Frequency of hematopoietic cells was determined by flow cytometry, number of cells was determined out of total MNC counts, and fold expansion was calculated. One-way ANOVA multiple comparisons was used; n = 4 BM donor mice per genotype, with 2 technical replicates per donor. Data are presented as mean values +/- SEM. h, HSPC frequency as determined by flow cytometry. One-way ANOVA multiple comparisons was used; n = 4 BM donor mice per genotype, with 2 technical replicates per donor. Data are presented as mean values +/- SEM. i, Proposed model for Fli-1 endorsed HSPC activation via Notch/Vegfa pathways mediated crosstalk with the vascular niche. Fli-1 in HSPCs, transcriptionally presets Notch pathway-promoting external and internal elements while also transcriptionally promoting Vegfa expression. Vegfa enlists an activation supportive vascular niche by stimulating ECs to elevate the expression of Notch ligands. Reciprocal niche-mediated activation of Notch receptors presented by HSPCs, endorse downstream Notch signaling, promoting HSPC regenerative activation and expansion. Created with BioRender.com.
Extended Data Fig. 8 Enforcing Notch1 signaling in Fli-1 deficient HSPCs restores the active-HSC sub-population.
WT, Fli-1ROSAΔ, Fli-1ROSAΔN1-ICiOE and N1-ICiOE BM LSK HSPCs were isolated and expanded in co-culture with a vascular niche. Collected cells were analyzed by flow cytometry and/or flow sorted for LSK HSPCs which were transcriptionally analyzed by scRNA-seq. a, BrdU was supplemented to co-culture media on day 4 post 4-OHT induction and cells were analyzed after co-cultures were terminated. Frequency of BrdU+ LSK HSPC was determined by flow cytometry. One-way ANOVA multiple comparisons was used; n = 5 BM donor mice per genotype, with 2 technical replicates per donor. Data are presented as mean values +/- SEM. b, c, Cells in co-culture were analyzed after 4 days post 4-OHT induction. b, Frequency of Cdk6+ LSK HSPC was determined by flow cytometry. One-way ANOVA multiple comparisons was used; n = 5 BM donor mice per genotype, with 2 technical replicates per donor. Data are presented as mean values +/- SEM. c, Representative flow cytometry histograms for Cdk6 gated from LSK HSPCs. Histogram plot bars indicate the gates for the positive fluorescent signal. d,e, Statistics for the relative differences in cell proportions in per cluster were calculated for all possible pairwise combinations for the four geneteic phenotype conditions as a permutation test (n = 1,000, see Supplementary Data 7). d, Compositional analysis-based bar plot presentation of frequency of cells for each genetic phenotype per cluster. e, Compositional analysis-based bar plot presentation of frequency of cells in selected HSC clusters 7 and 15 only per genetic phenotype. f, Gene ontology (GO) categories of biological processes enrichment for differentially expressed genes in scRNA-seq analyses of HSC Cluster 15 vs. HSC Cluster 7, presented in a bar plot. Positively enrichment scored processes are enriched in HSC cluster 15 while negatively enrichment scored processes are enriched in HSC cluster 7.
Extended Data Fig. 9 Notch1-driven developmental skewing towards the lymphoid T-cell lineage and T-cell leukemia is attenuated by Fli-1 deficiency.
WT, Fli-1ROSAΔ, Fli-1ROSAΔN1-ICiOE, and N1-ICiOE BM LSK HSPCs were isolated and expanded. At the end of the expansion step in co-culture, cells were collected and hematopoietic LSK HSPCs were competitively transplanted with total SJL BM cells into lethally irradiated SJL mice a. Survival plot for mice transplanted with WT, Fli-1ROSAΔ, Fli-1ROSAΔN1-ICiOE, and N1-ICiOE expanded LSK HSPCs. Gehan-Breslow-Wilcoxon test was used; n = 11-14 mice per genotype group. b-h, At week 5 post transplantation of WT and N1-ICiOE BM LSK HSPCs blood was collected and hematological parameters were scored using HESKA hematological analyzer or by flow cytometry. Data are presented as mean values +/- SEM. b, White blood cell (WBC), c, red blood cell (RBC), and d, Platelet counts are presented per 1 µL of blood. e, Frequency of chimerism as determined by flow cytometry. f, Representative flow cytometry dot plots for CD45.2 and CD45.1 congenic markers for WT (upper) N1-ICiOE (lower) transplanted mice. g, Frequency of transplanted chimeric cell distribution among hematopoietic lineages as determined by flow cytometry. h, Representative flow cytometry dot plots for CD3 and B220 lymphoid markers for WT (left) N1-ICiOE (right) transplanted mice. Unpaired two tailed t-test was used; n = 8 per WT or N1-ICiOE. i-k, Frequency of chimerism among myeloid cells, B-cells, and T-cells as determined by flow cytometry. One-way ANOVA multiple comparisons was used; n = 8 recipient mice per genotype. Data are presented as mean values +/- SEM.
Extended Data Fig. 10 Transcriptional comparison distinguishes distinct activation states for neonatal and adult HSPCs.
a, Violin plot presentation of for cycle scores (S phase and G2/M phases) per cellular source (CB or mPB), for HSPC clusters in analysis presented in Fig. 6a-d. Two sided Wilcoxon Rank Sum Test was used to determine p-Values (indicated per cluster). Normality was rejected after running the Shapiro-Wilks test for cell cycle scores among all HSPC sub-types. Events n=per cluster: Late Ery CB = 323 mPB=44, Early Ery CB = 1397 mPB=520, GMP CB = 334 mPB=41, lymphocyte CB = 85 mPB=19, BaEoMa CB = 215 mPB=36, Plasma CB = 17 mPB=90, HSC_MPP CB = 1148 mPB=1757, and LMPP CB = 2288 mPB=1193. b, Gene ontology (GO) categories of reactome enrichment for differentially expressed genes in scRNA-seq analyses of cells from CB or mPB source in the HSC\MPP Cluster, presented in a bar plot. Positively enrichment scored processes are enriched in CB derived HSC\MPP cells while negatively enrichment scored processes are enriched in mPB derived HSC\MPP cells. c, Gene ontology (GO) categories of Hallmark signatures pathways enrichment for differentially expressed genes in RNA-seq analyses of sorted HSCs from CB or mPB source, presented in a bar plot. Positively enrichment scored processes are enriched in mPB HSCs while negatively enrichment scored processes are enriched in CB HSCs. d, Collected cells from 2 day co-cultures were stained and analyzed by flow cytometry to determine frequency of NOTCH1 expressing CD34+ HSPCs. Unpaired two tailed t-test was used; n = 6 donors, each point represents an average of 2 technical replicates. Representative NOTCH1 flow cytometry histogram plots were gated from CD34+ HSPCs. Histogram plot bars indicate the gates for the positive fluorescent signal. Data are presented as mean values +/- SEM. e, Collected cells from 2 day co-cultures were stained and analyzed by flow cytometry to determine frequency of VEGFA expressing CD34+ HSPCs. Unpaired two tailed t-test was used; n = 6 donors, each point represents an average of 2 technical replicates. Representative VEGFA flow cytometry histogram plots were gated from CD34+ HSPCs. Histogram plot bars indicate the gates for the positive fluorescent signal. Data are presented as mean values +/- SEM.
Supplementary information
Supplementary Information
Supplementary Methods and Supplementary Tables 1 and 2
Supplementary Data 1
snRNA-seq differential gene expression in distinct HSPC subclusters.
Supplementary Data 2
Full heat map representation of differential chromVAR motif activity in distinct HSPC subclusters by averaged z-score. All predicted TF motifs names are presented.
Supplementary Data 3
snATAC-seq differential chromVAR motif activity in distinct HSC/MPP subclusters and snRNA-seq differential gene expression in distinct HSC/MPP subclusters. GO for biological processes is presented for each set of snRNA/ATAC-seq analyses.
Supplementary Data 4
RNA-seq differential gene expression in Fli-1ROSAΔ versus WT HSPCs.
Supplementary Data 5
ATAC-seq differential gene peaks calling in Fli-1ROSAΔ versus WT HSPCs.
Supplementary Data 6
H3K27ac ChIP–seq differential gene peaks calling in Fli-1ROSAΔ versus WT HSPCs.
Supplementary Data 7
Statistical comparison per genotype per cluster for scRNA-seq cluster compositional analysis presented in Extended Data Fig. 8d,e.
Supplementary Data 8
scRNA-seq differential gene expression in 4-month engrafted Fli-1ROSAΔ N1-ICiOE versus WT HSPC populations.
Supplementary Data 9
scRNA-seq differential gene expression in CB HSC-MPP cluster versus mPB HSC-MPP cluster.
Supplementary Data 10
RNA-seq differential gene expression in CB HSC versus mPB HSCs.
Supplementary Data 11
Targeted DNA-seq comparative analysis and statistics for control versus FLI-1 modRNA treated mPB HSPCs after expansion in co-culture.
Supplementary Video 1
Intravital representative video of BM homed HSPCs. DiD-labeled Fli-1ROSAΔ HSPCs (red arrow) and autofluorescence (red). DiI-labeled WT HSPCs (white arrow), autofluorescence and dextran-labeled blood vessels (blue). For bone, second-harmonic generation of collagen shown in green. Scale bar, 50 µm. z-step size, 3 µm.
Supplementary Video 2
Intravital representative video of vessel wedged HSPCs. DiI-labeled Fli-1ROSAΔ HSPCs (yellow arrows), autofluorescence and dextran-labeled blood vessels (blue). For bone, second-harmonic generation of collagen shown in green. Scale bar, 50 µm. z-step size, 3 µm.
Supplementary Video 3
Representative video of expending HSPCs in culture. WT (left) and Fli-1ROSAΔ (right) BM HSPCs were isolated and introduced into co-culture with E4orf1 vascular niche cells as described in Supplementary Fig. 5a. Time tab indicates the hours that passed since the addition of 4-OHT into cultures. Scale bar, 500 µm.
Supplementary Video 4
Representative video of expending HSPCs in culture. WT (left) and Fli-1ROSAΔ (right) BM HSPCs were isolated and introduced into co-culture with E4orf1 vascular niche cells as described in Supplementary Fig. 5a. Time tab indicates the hours that passed since the addition of 4-OHT into cultures. Scale bar, 100 µm.
Supplementary Video 5
Representative video of human adult mPB HSPCs in culture. Transduced control (upper) and FLI-1 modified-RNA (lower) human adult mPB HSPCs were isolated and introduced into co-culture with E4orf1 vascular niche cells. Time tab indicates the hours that passed since introduction of HSPCs into co-cultures. Scale bar, 200 µm.
Source data
Source Data Figs. 1–8 and Extended Data Figs. 1–10
Source data file for all data presented in graphs within the figures and extended data figures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Itkin, T., Houghton, S., Schreiner, R. et al. Transcriptional activation of regenerative hematopoiesis via microenvironmental sensing. Nat Immunol 26, 378–390 (2025). https://doi.org/10.1038/s41590-025-02087-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-025-02087-w